- For US Healthcare Providers
- Full Prescribing Information
FOR ADULTS WITH ACTIVE AS
SIMPONI ARIA® IS THE FIRST AND ONLY ANTI-TNFα BIOLOGIC WITH IMPROVEMENT IN BOTH PHYSICAL AND MENTAL COMPONENT SUMMARY SCORES OF SF-36 IN THE LABEL
ASAS20 response at Week 16 (primary endpoint): 73% of patients receiving SIMPONI ARIA® achieved ASAS20 response vs 26% of patients receiving placebo (P<0.001)1,2
SF-36 is a 36-item short-form health survey for patients. This instrument yields an 8-domain profile of functional health and well-being scores, as well as psychometrically based PCS and MCS scores.
Mean change from baseline for patients receiving SIMPONI ARIA® vs patients receiving placebo
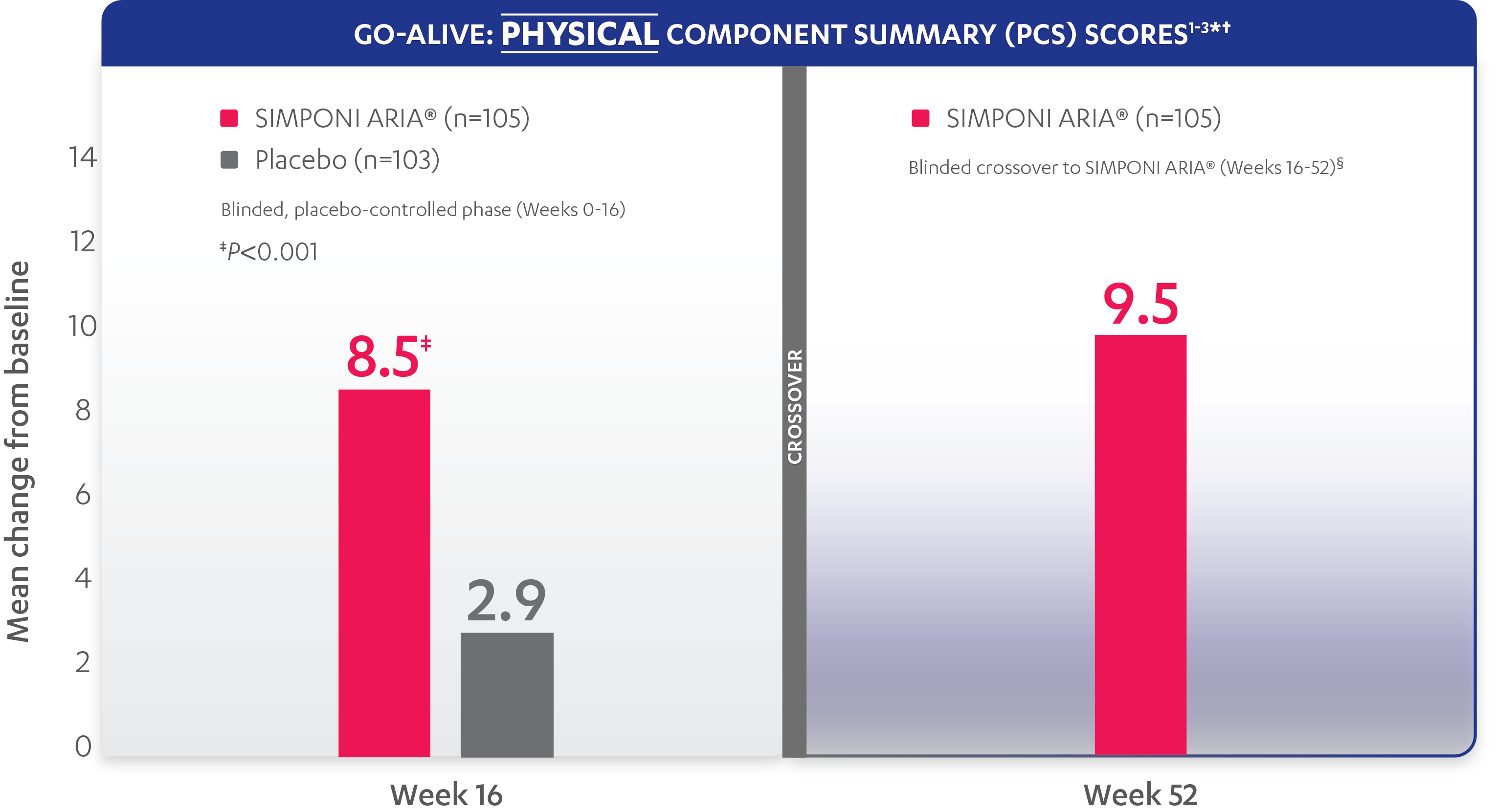
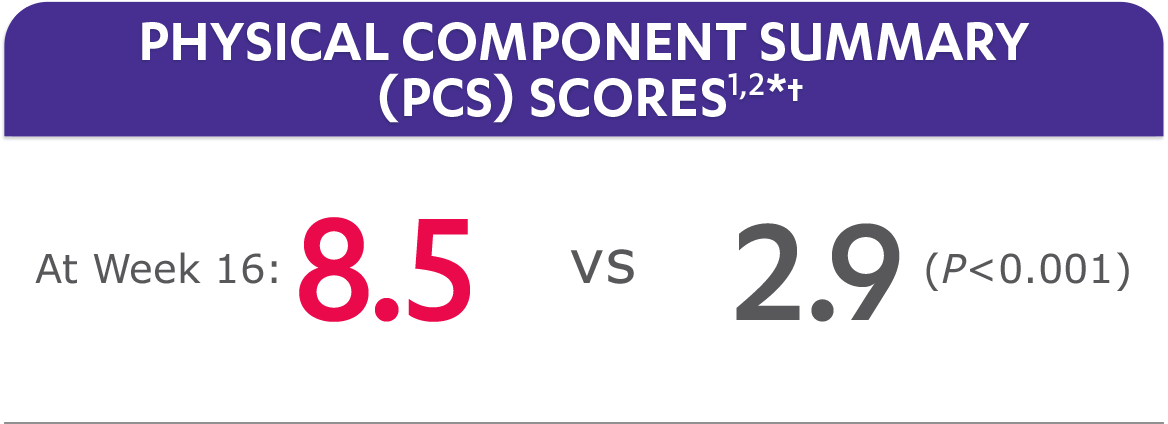
In the GO-ALIVE study, a change from baseline in SF-36 score, including PCS and MCS, of ≥5 points was considered clinically meaningful.
Mean change from baseline for patients receiving SIMPONI ARIA® vs patients receiving placebo
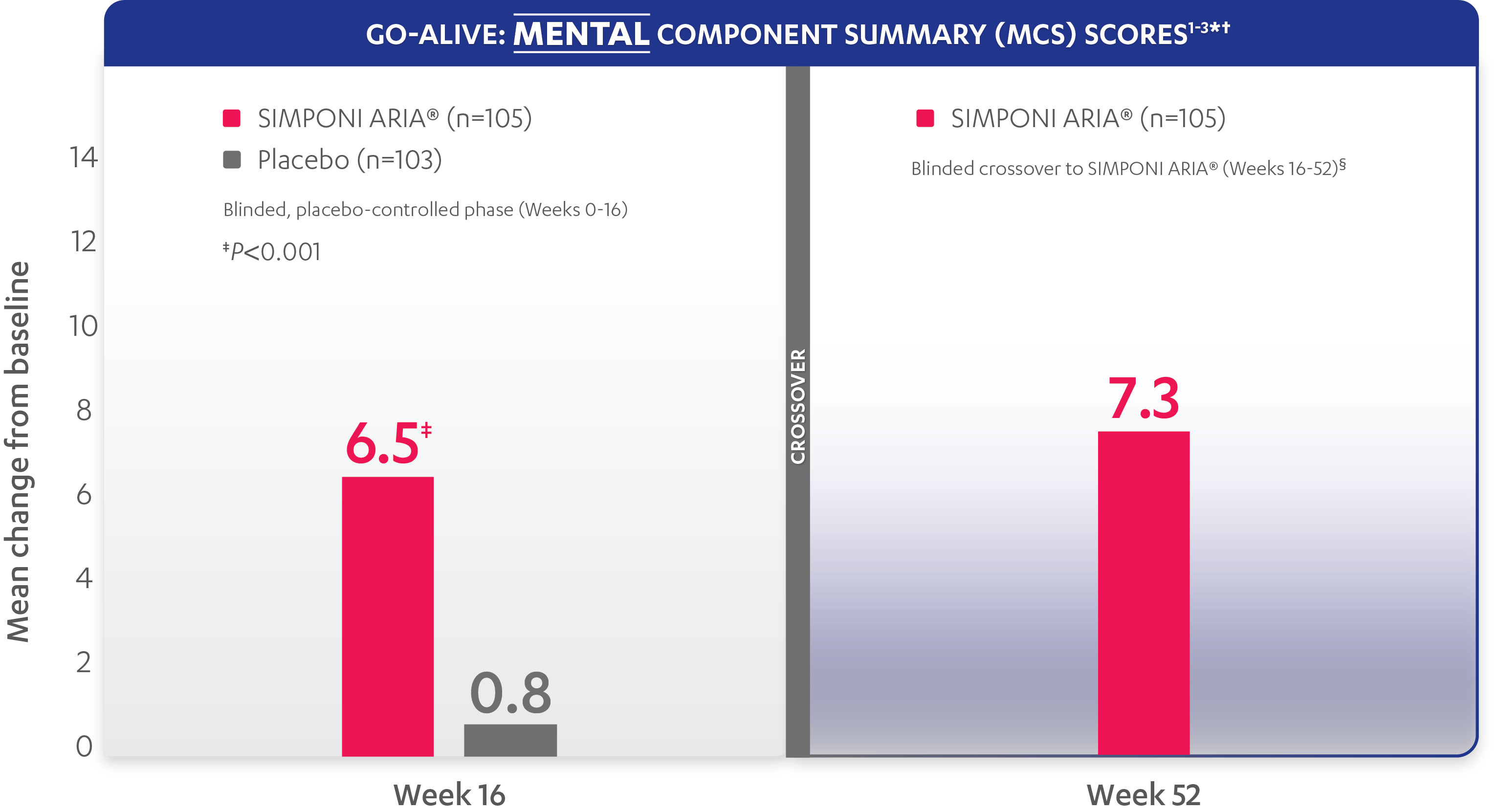
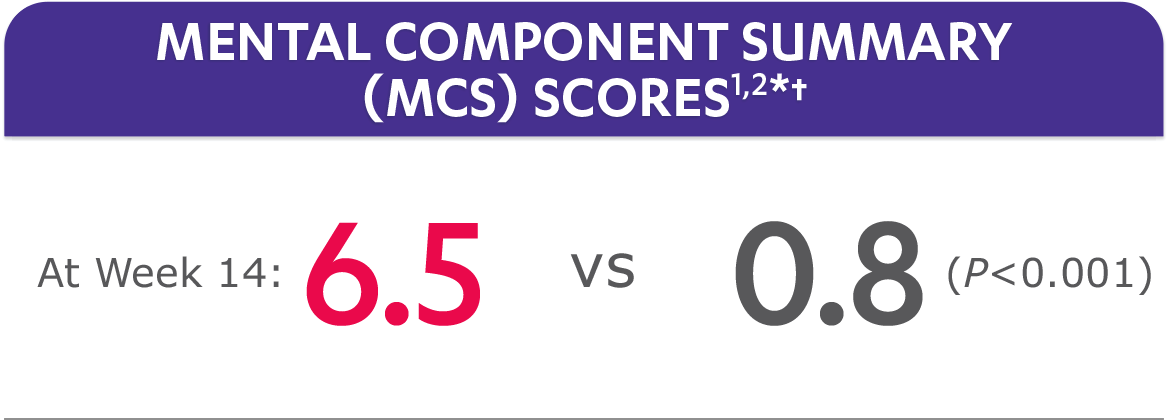
In the GO-ALIVE study, a change from baseline in SF-36 score, including PCS and MCS, of ≥5 points was considered clinically meaningful.
*The SF-36 is a validated questionnaire. The same patients may not have responded at each timepoint.
†Change from baseline in SF-36 is based on imputed data using LOCF for missing data.
§After Week 16, patients and doctors knew that all patients were on SIMPONI ARIA® (blinded active treatment), which may have affected the results.
The 8 multi-item domains of the SF-36 instrument are2:
Physical health domains
- Limitations in physical functioning due to health problems
- Limitations in usual role activities due to physical health problems
- Bodily pain
- General health perception
Mental health domains
- Limitations in social functioning due to physical or mental health problems
- Limitations in usual role activities due to personal or emotional problems
- Vitality (energy and fatigue)
- General mental health (psychological distress and well-being)
Study design: GO-ALIVE was a global, multicenter, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of SIMPONI ARIA® compared with placebo in 208 adult patients with active AS with an inadequate response or intolerance to NSAIDs. Patients had a diagnosis of definite AS for at least 3 months according to modified New York criteria. Patients had symptoms of active disease [Bath AS Disease Activity Index (BASDAI) ≥4, VAS for total back pain of ≥4, on scales of 0 to 10 cm (0 to 100 mm), and an hsCRP level of ≥0.3 mg/dL (3 mg/L)]. At Week 0, patients were randomized in a 1:1 ratio to 1 of 2 treatment groups. Subjects in the placebo group (n=103) were randomized to receive IV placebo infusions at Weeks 0, 4, and 12. At Week 16, these patients were crossed over to SIMPONI ARIA® and received administrations at Weeks 16, 20, and q8w thereafter through Week 52. Patients in the SIMPONI ARIA® group (n=105) were randomized to receive SIMPONI ARIA® 2-mg/kg infusions at Weeks 0, 4, and 12. These patients received a placebo infusion at Week 16 to maintain the treatment blind and continued to receive SIMPONI ARIA® infusions at Week 20 and q8w thereafter through Week 52. Patients were allowed to continue stable doses of concomitant MTX, SSZ, hydroxychloroquine (HCQ), low dose oral corticosteroids (equivalent to ≤10 mg of prednisone per day), and/or NSAIDs during the trial. The primary endpoint was the percentage of patients achieving ASAS20 response at Week 16.1

INFUSION EXPERIENCE
SIMPONI ARIA® offers an efficient 30-minute infusion.
DOSING CALCULATOR
Calculate an adult patient's dose of SIMPONI ARIA® based on weight.
ACCESS AND AFFORDABILITY
View first-line coverage rates for SIMPONI ARIA®.
