Choose A Condition:
Please Select
Adverse events (AEs) through Week 601,2
| SIMPONI ARIA® | |
| Number of patients, n | 204 |
| Mean follow-up, weeks | 51.8 |
| Patients with ≥1 AE, % (n) | 55.4% (113) |
| Patients with ≥1 serious AE, % (n) | 3.4% (7) |
| Discontinuation rate due to AEs, % (n) | 2.0% (4) |
| Patients with ≥1 infection, % (n) | 32.8% (67) |
| Patients with ≥1 serious infection, % (n) | 1.5% (3) |
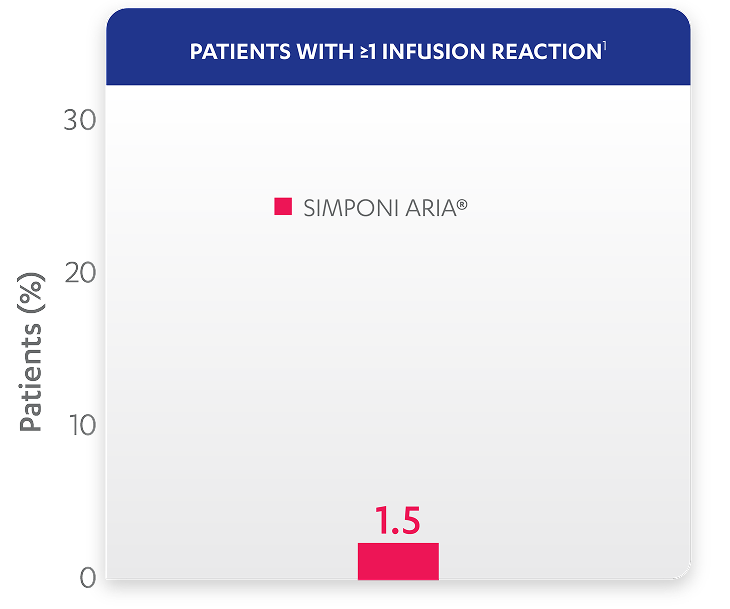
| Patients with ≥1 infusion reaction, % (n) | 1.5% (3) |
| Most common AEs (occurring in ≥5% of patients treated with SIMPONI ARIA®) | |
| Nasopharyngitis % (n) | 11.8% (24) |
| Upper respiratory tract infection, % (n) | 7.4% (15) |
| Alanine aminotransferase (ALT) increased, % (n) | 5.9% (12) |
Infusion reactions reported through Week 601
AE=adverse events; AS=ankylosing spondylitis.
References: 1. Data on file. Johnson & Johnson. 2. SIMPONI ARIA® [Prescribing Information]. Horsham, PA: Johnson & Johnson.