Choose A Condition:
Please Select
Controlled and uncontrolled phase (Week 60)1,2
| Combined SIMPONI ARIA® +/- MTX | |
| Number of patients, n | 460 |
| Mean follow-up, weeks | 47.2 |
| Patients with ≥1 AE, % (n) | 50.9 (234) |
| Patients with ≥1 serious AE, % (n) | 5.2 (24) |
| Discontinuation rate due to AEs, % (n) | 3.7 (17) |
| Deaths, % (n) | 0.2 (1) |
| Patients with ≥1 infection, % (n) | 22.8 (105) |
| Patients with ≥1 serious infection, % (n) | 2.2 (10) |
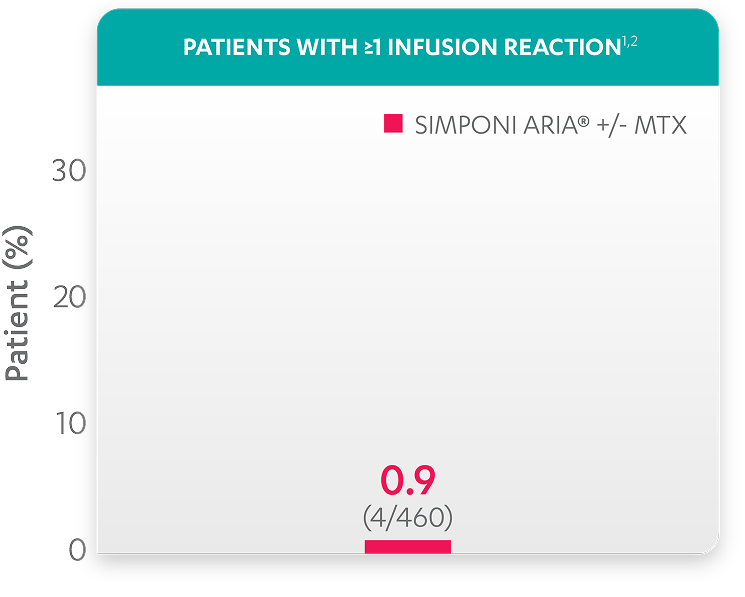
| Patients with ≥1 infusion reaction, % (n) | 0.9 (4) |
| Patients with active tuberculosis, % (n) | 0.4 (2) |
| Patients with ≥1 malignancy, % (n) | 0.4 (2) |
| Patients with ≥1 demyelinating event, % (n) | 0.2 (1) |
| Most common AEs occurring in ≥5% of patients treated with SIMPONI ARIA® +/- MTX, %(n) | |
| Nasopharyngitis | 5.0 (23) |
| ALT increased | 8.3 (38) |
| AST increased | 6.7 (31) |
INFUSION REACTIONS WITH SIMPONI ARIA® vs PLACEBO-CONTROLLED PATIENTS THROUGH WEEK 601,2
AE=adverse events; ALT=alanine aminotransferase; AST=aspartate aminotransferase; MTX=methotrexate; PsA=psoriatic arthritis.
References: 1. Data on file. Johnson & Johnson. 2. Husni ME, Kavanaugh A, Murphy F, et al. Efficacy and safety of intravenous golimumab through one year in patients with active psoriatic arthritis. Arthrit Care Res. 2006;45(6):885-889.