PATIENT-REPORTED OUTCOMES
SIMPONI ARIA® improvement in fatigue data as measured by FACIT-F in a clinical trial.
LEARN MOREACR20 response at Week 14 (primary endpoint): 59% of patients receiving SIMPONI ARIA® + MTX achieved ACR20 response vs 25% of patients receiving placebo + MTX (P<0.001)1,2
HAQ-DI* score at Week 14: the mean improvement from baseline in HAQ-DI score was 0.50 for patients receiving SIMPONI ARIA® + MTX (n=395) vs 0.19 for patients receiving placebo + MTX (n=197) (P<0.001)1,2
*A reduction in HAQ-DI score of ≥0.25 is clinically meaningful. The HAQ-DI is a validated questionnaire. It is scored from 0 (no disability) to 3 (completely disabled).
ACR20=20% improvement in American College of Rheumatology criteria; HAQ-DI=health assessment questionnaire-disability index; ITT=intention-to-treat; MTX=methotrexate; RA=rheumatoid arthritis; SF-36=36-item short-form survey.
SF-36 is a 36-item short-form health survey for patients. This instrument yields an 8-domain profile of functional health and well-being scores, as well as psychometrically based physical component summary (PCS) and mental component summary (MCS) scores.3
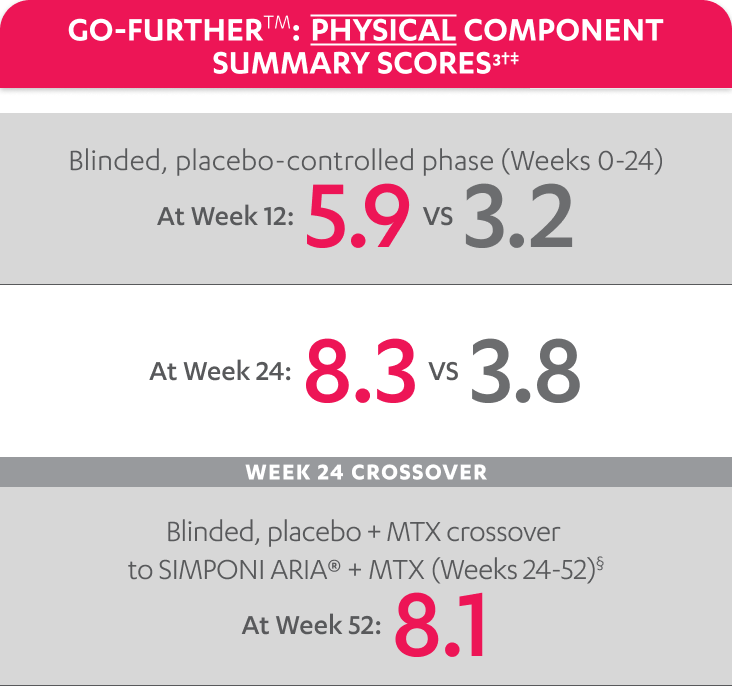
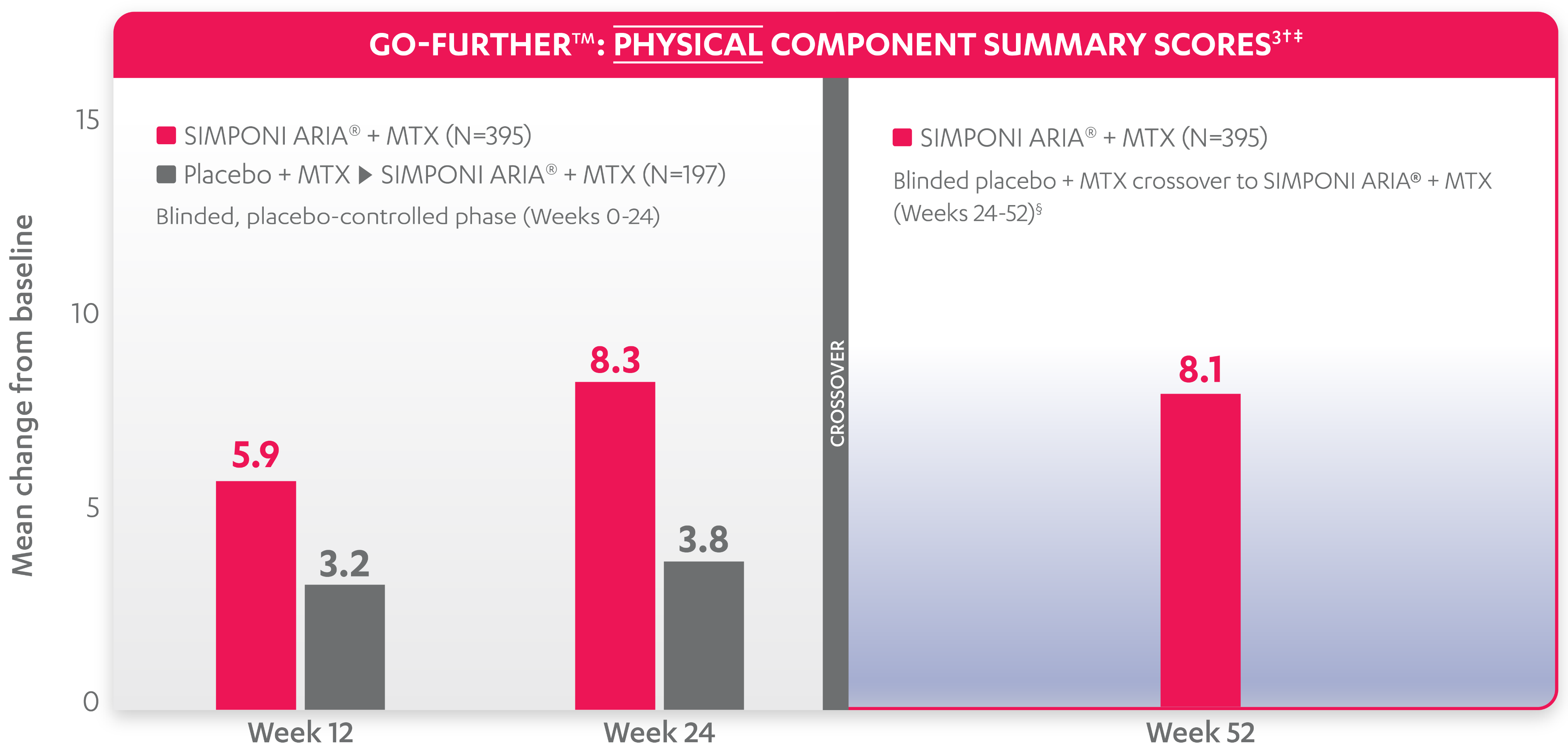
SF-36 PCS scores were not adjusted for multiplicity. Therefore, statistical significance has not been established.
In the GO-FURTHER™ study, a change from baseline in SF-36 score, including PCS and MCS, of ≥5 points was considered clinically meaningful.
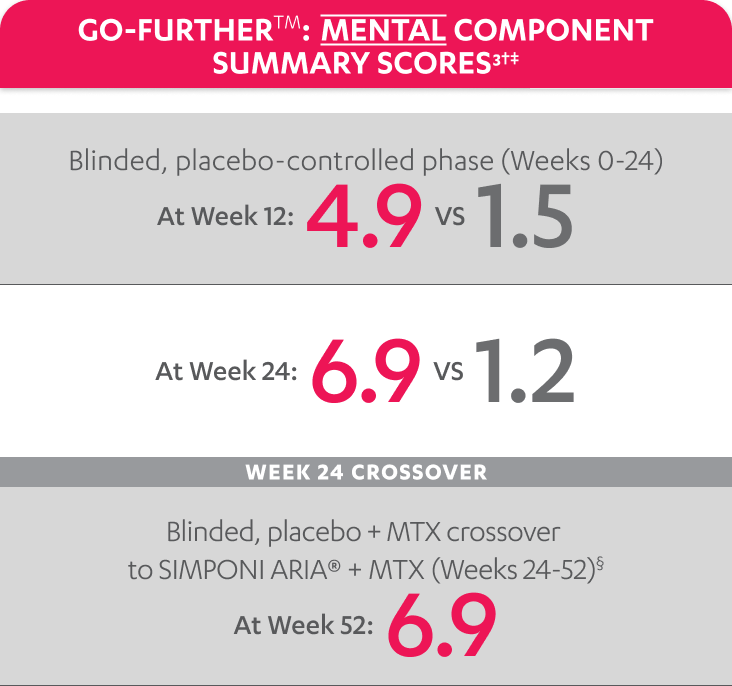
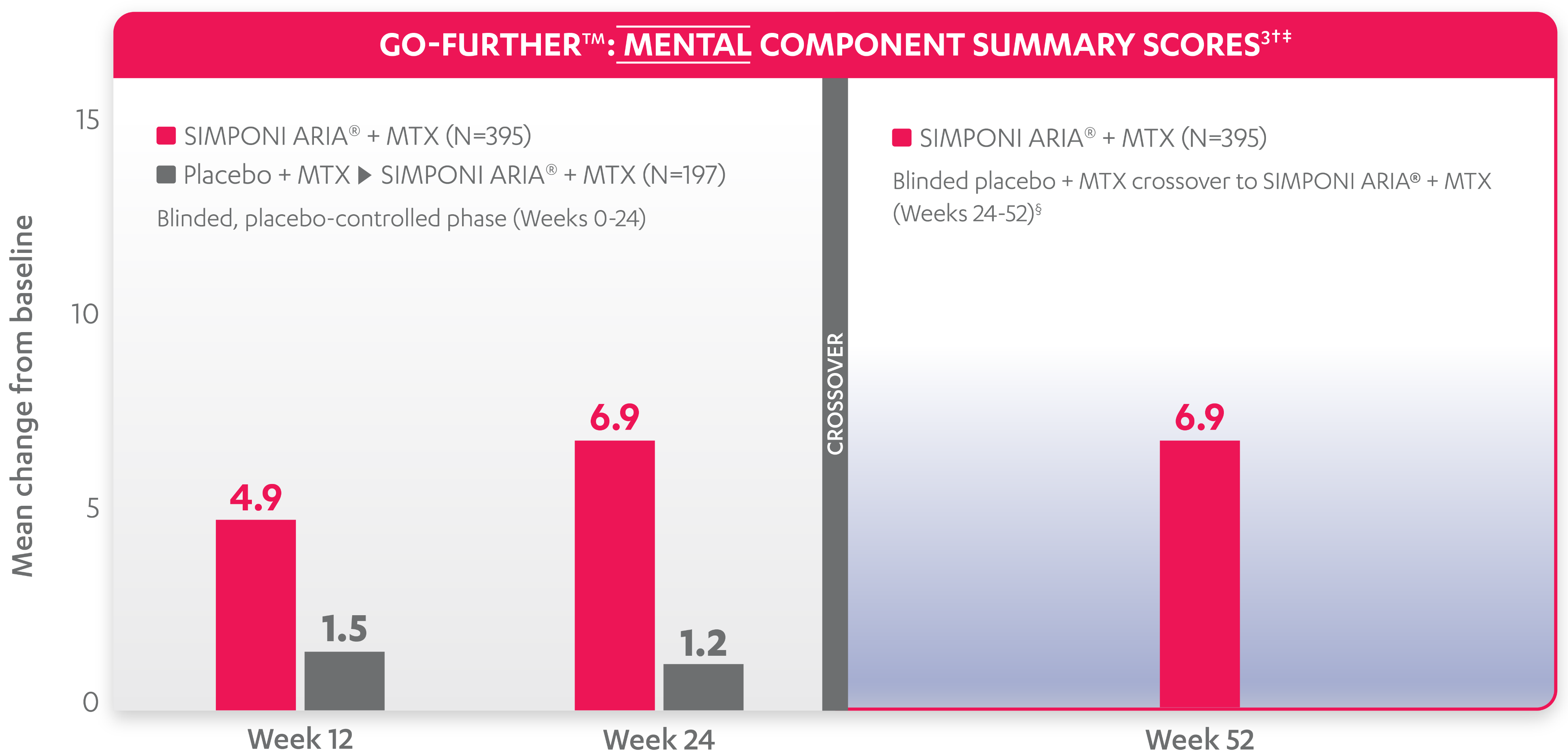
SF-36 MCS scores were not adjusted for multiplicity. Therefore, statistical significance has not been established.
In the GO-FURTHER™ study, a change from baseline in SF-36 score, including PCS and MCS, of ≥5 points was considered clinically meaningful.
†The randomized (intent-to-treat) population was used for these analyses.
‡SF-36 is a validated questionnaire. The same patients may not have responded at each time point. Mean change from baseline in SF-36 score is based on imputed data using the last observation carried forward for missing data.
§After Week 24, all sponsor personnel were unblinded to subject-level data, which may have affected results. Additionally, 9 patients were inadvertently unblinded prior to Week 24, which may also have affected results.
The 8 multi-item domains of the SF-36 instrument are3:
Physical health domains
Mental health domains
Study design: GO-FURTHER™ was a global, multicenter, randomized, double-blind, placebo-controlled study in 592 adult patients who had moderately to severely active RA despite a stable dose of MTX (15-25 mg/week) for ≥3 months and who had not been previously treated with an anti-TNF agent. Moderately to severely active RA was defined as ≥6 swollen joints (out of 66 total) and ≥6 tender joints (out of 68 total), RF positive and/or anti-CCP antibody positive, and CRP ≥1.0 mg/dL. Patients were randomized to receive SIMPONI ARIA® 2 mg/kg + MTX (n=395) or placebo + MTX (n=197) as a 30-minute IV infusion at Weeks 0 and 4, and then q8w through Week 100. At Week 16, patients in the placebo + MTX group with <10% improvement from baseline in both swollen joint count and tender joint count began receiving SIMPONI ARIA® 2 mg/kg beginning with an induction regimen at Weeks 16 and 20, followed by maintenance infusions q8w in a blinded manner. At Week 24, all patients remaining in the placebo + MTX group began receiving SIMPONI ARIA® 2 mg/kg beginning with an induction regimen at Weeks 24 and 28, followed by maintenance infusions q8w in a blinded manner. All patients continued to receive MTX. The primary endpoint was the percentage of patients achieving an ACR20 response at Week 14.3
CCP=cyclic citrullinated peptide; CRP=C-reactive protein; FACIT-F=Functional Assessment of Chronic Illness Therapy–fatigue; ITT=intention-to-treat; IV=intravenous; q8w=every 8 weeks; RA=rheumatoid arthritis; RF=rheumatoid factor; TNF=tumor necrosis factor.
PATIENT-REPORTED OUTCOMES
SIMPONI ARIA® improvement in fatigue data as measured by FACIT-F in a clinical trial.
LEARN MOREReferences: 1. SIMPONI ARIA® (golimumab) [Prescribing Information]. Horsham, PA: Johnson & Johnson. 2. Weinblatt ME, Bingham CO III, Mendelsohn AM, et al. Intravenous golimumab is effective in patients with active rheumatoid arthritis despite methotrexate therapy with responses as early as week 2: results of the phase 3, randomised, multicentre, double-blind, placebo-controlled GO-FURTHER trial. Ann Rheum Dis. 2013;72:381-389. 3. Data on file. Johnson & Johnson.