Choose A Condition:
Please Select
FOR ADULTS WITH MODERATELY TO SEVERELY ACTIVE RA, IN COMBINATION WITH MTX
ACR20 RESPONSE RATES OVER TIME
BLINDED, PLACEBO-CONTROLLED PHASE (WEEKS 0-24)†
59%

25%

33%

12%

63%

32%

ACR20 responses at Week 2 and Week 24 were not adjusted for multiplicity. Therefore, statistical significance has not been established.
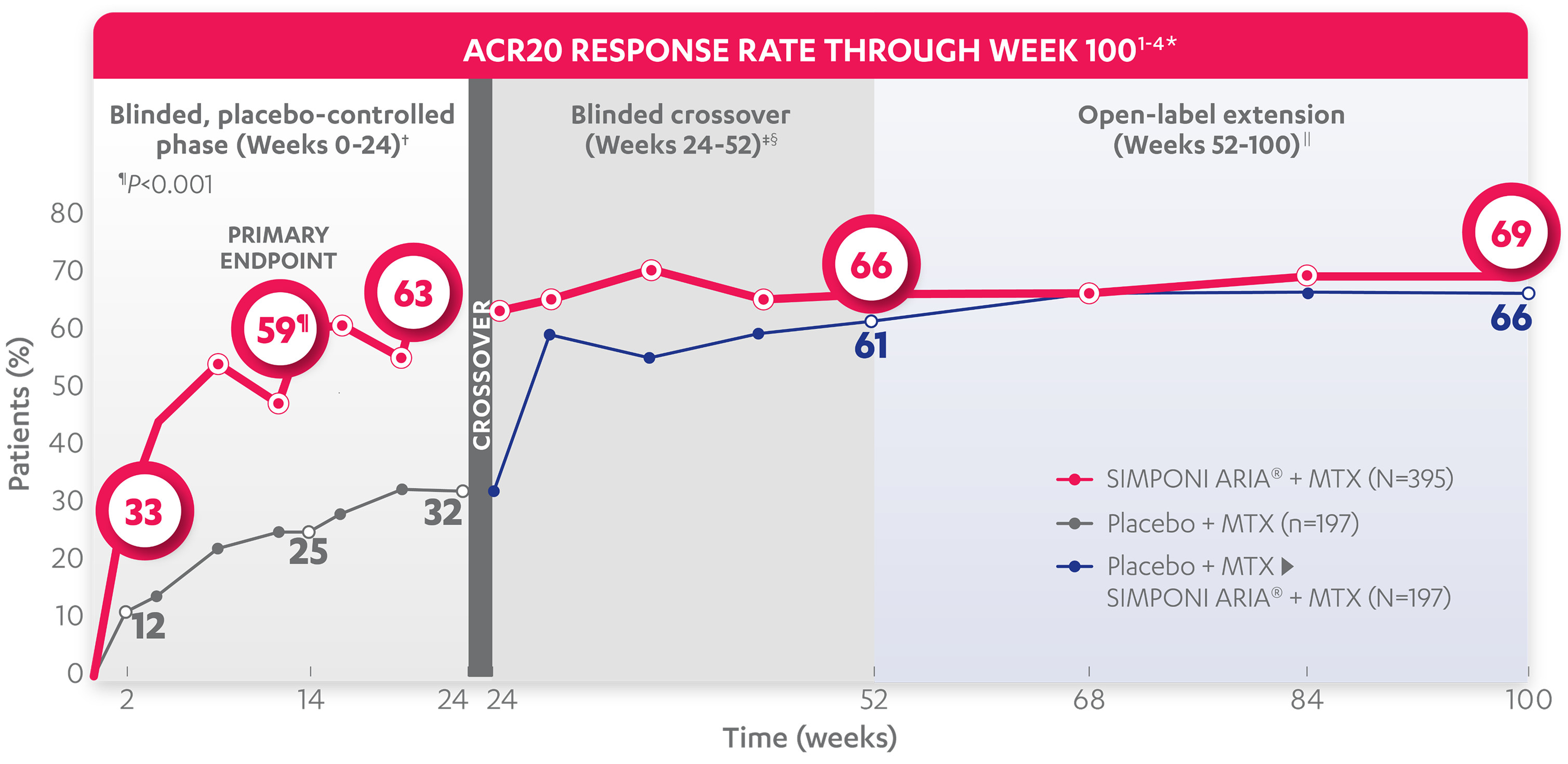
66%
61%
69%
66%
*The same patients may not have responded at each time point.
†In this ITT analysis, patients were considered to be nonresponders if they experienced any of the following: increased MTX dose above baseline for RA; initiated treatment with DMARDs (including commercial MTX), systemic immunosuppressives, and/or biologics for RA; initiated treatment with oral corticosteroids for RA above baseline dose or received IV or intramuscular corticosteroids for RA; or discontinued treatment due to an unsatisfactory therapeutic effect. Last observation carried forward rules were applied to missing data. The patients who early escaped at Week 16 also had Week 16 data carried forward up through Week 24.
‡At Week 24, all remaining patients in the placebo + MTX group began receiving SIMPONI ARIA® + MTX in a blinded manner.
§In this ITT analysis, all rules were nullified and reapplied after Week 24. Patients were considered to be nonresponders if they discontinued treatment due to an unsatisfactory therapeutic effect.
||At Week 52, all study personnel were unblinded to subject-level data.
ACR50 RESPONSE RATES OVER TIME
BLINDED, PLACEBO-CONTROLLED PHASE (WEEKS 0-24)†
30%

9%

35%

13%

ACR50 responses at Week 2 and Week 24 were not adjusted for multiplicity. Therefore, statistical significance has not been established.
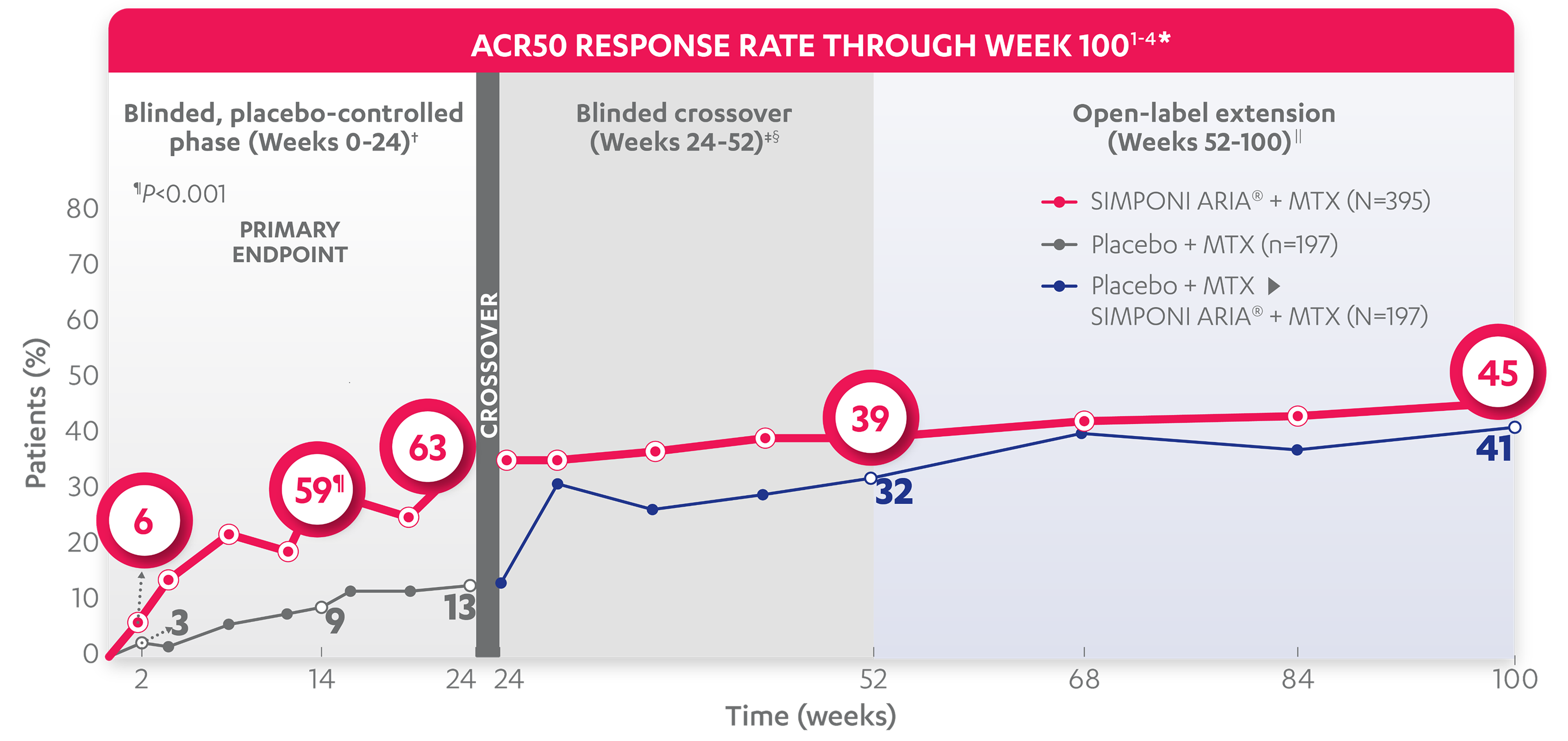
39%
32%
45%
41%
*The same patients may not have responded at each time point.
†In this ITT analysis, patients were considered to be nonresponders if they experienced any of the following: increased MTX dose above baseline for RA; initiated treatment with DMARDs (including commercial MTX), systemic immunosuppressives, and/or biologics for RA; initiated treatment with oral corticosteroids for RA above baseline dose or received IV or intramuscular corticosteroids for RA; or discontinued treatment due to an unsatisfactory therapeutic effect. Last observation carried forward rules were applied to missing data. The patients who early escaped at Week 16 also had Week 16 data carried forward up through Week 24.
‡At Week 24, all remaining patients in the placebo + MTX group began receiving SIMPONI ARIA® + MTX in a blinded manner.
§In this ITT analysis, all rules were nullified and reapplied after Week 24. Patients were considered to be nonresponders if they discontinued treatment due to an unsatisfactory therapeutic effect.
||At Week 52, all study personnel were unblinded to subject-level data.
ACR70 RESPONSE RATES OVER TIME
BLINDED, PLACEBO-CONTROLLED PHASE (WEEKS 0-24)†
12%

3%

18%

4%

ACR70 responses at Week 2 and Week 24 were not adjusted for multiplicity. Therefore, statistical significance has not been established.
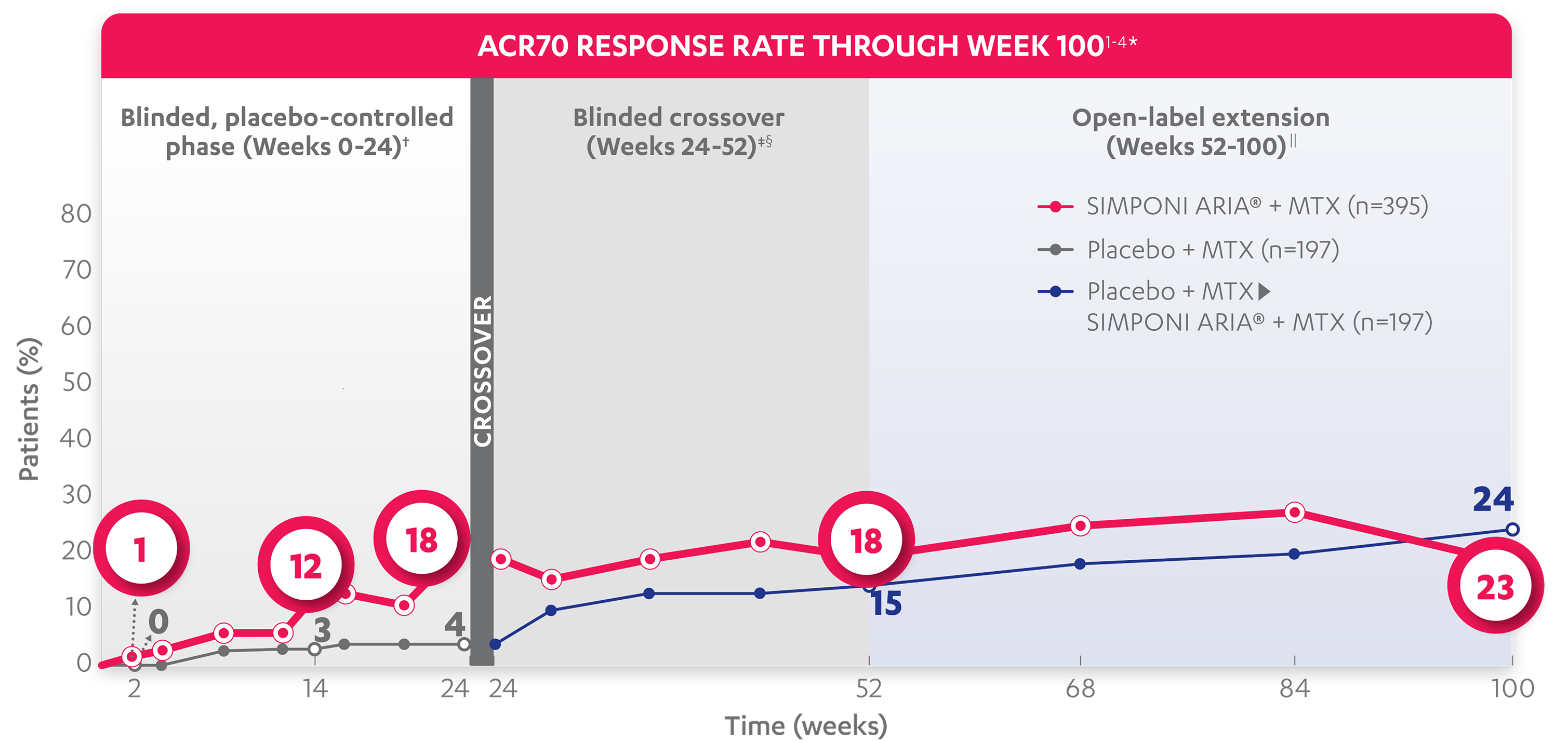
18%
15%
23%
24%
*The same patients may not have responded at each time point.
†In this ITT analysis, patients were considered to be nonresponders if they experienced any of the following: increased MTX dose above baseline for RA; initiated treatment with DMARDs (including commercial MTX), systemic immunosuppressives, and/or biologics for RA; initiated treatment with oral corticosteroids for RA above baseline dose or received IV or intramuscular corticosteroids for RA; or discontinued treatment due to an unsatisfactory therapeutic effect. Last observation carried forward rules were applied to missing data. The patients who early escaped at Week 16 also had Week 16 data carried forward up through Week 24.
‡At Week 24, all remaining patients in the placebo + MTX group began receiving SIMPONI ARIA® + MTX in a blinded manner.
§In this ITT analysis, all rules were nullified and reapplied after Week 24. Patients were considered to be nonresponders if they discontinued treatment due to an unsatisfactory therapeutic effect.
||At Week 52, all study personnel were unblinded to subject-level data.
References: 1. SIMPONI ARIA® (golimumab) [Prescribing Information]. Horsham, PA: Johnson & Johnson. 2. Data on file. Johnson & Johnson. 3. Weinblatt ME, Bingham CO III, Mendelsohn AM, et al. Intravenous golimumab is effective in patients with active rheumatoid arthritis despite methotrexate therapy with responses as early as week 2: results of the phase 3, randomised, multicentre, double-blind, placebo-controlled GO-FURTHER trial. Ann Rheum Dis. 2013;72:381-389. 4. Bingham CO III, Mendelsohn AM, Kim L, et al. Arthritis Care Res (Hoboken). 2015;67(12):1627-1636.