Choose A Condition:
Please Select
SIMPONI ARIA® IS THE FIRST FULLY HUMAN ANTI‑TNFα BIOLOGIC WITH IMPROVEMENT IN BOTH PHYSICAL AND MENTAL COMPONENT SUMMARY SCORES OF SF-36 IN THE LABEL1,2
ACR20 response at Week 14 (primary endpoint): 75% of patients receiving SIMPONI ARIA® +/- MTX achieved ACR20 response vs 22% of patients receiving placebo +/- MTX (P<0.001)1,3,4
SF-36 is a 36-item short-form health survey for patients. This instrument yields an 8-domain profile of functional health and well-being scores, as well as psychometrically based physical component summary (PCS) and
mental component summary (MCS) scores.4
SF-36 is a 36-item short-form health survey for patients. This instrument yields an 8-domain profile of functional health and well-being scores, as well as psychometrically based
physical component summary (PCS) and mental component summary (MCS) scores.4
Mean change from baseline for patients receiving SIMPONI ARIA® +/- MTX (n=233) vs patients receiving placebo +/- MTX (n=222)3-5
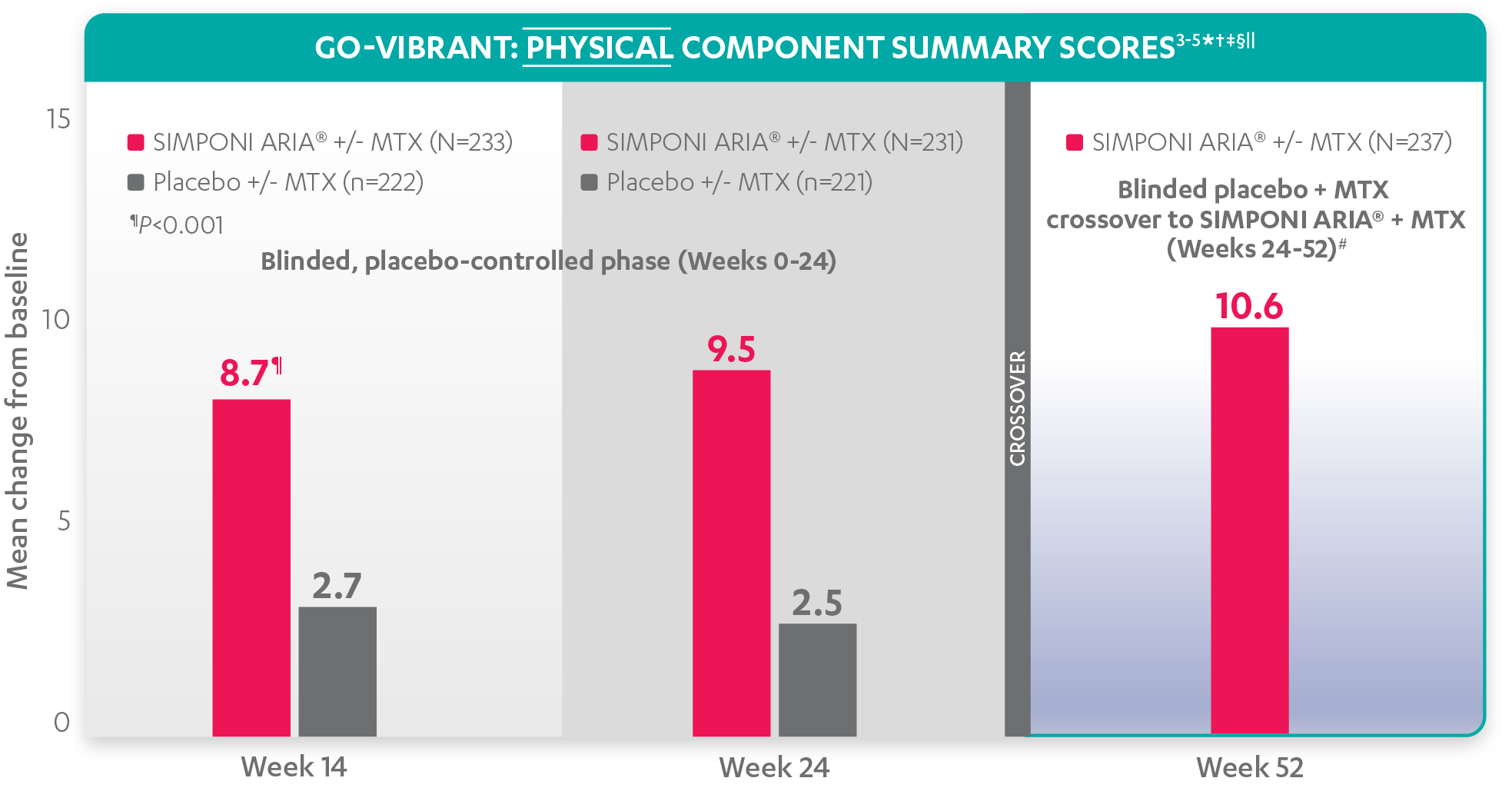

SF-36 PCS scores at Week 24 were not adjusted for multiplicity. Therefore, statistical significance has not been established.
In the GO-VIBRANT study, a change from baseline in SF-36 score, including PCS and MCS, of ≥5 points was considered clinically meaningful.
Mean change from baseline for patients receiving SIMPONI ARIA® +/- MTX (n=233) vs patients receiving placebo +/- MTX (n=222)3-5
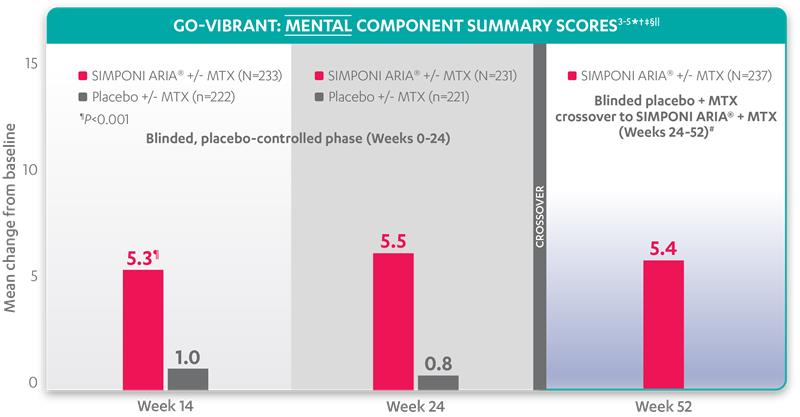
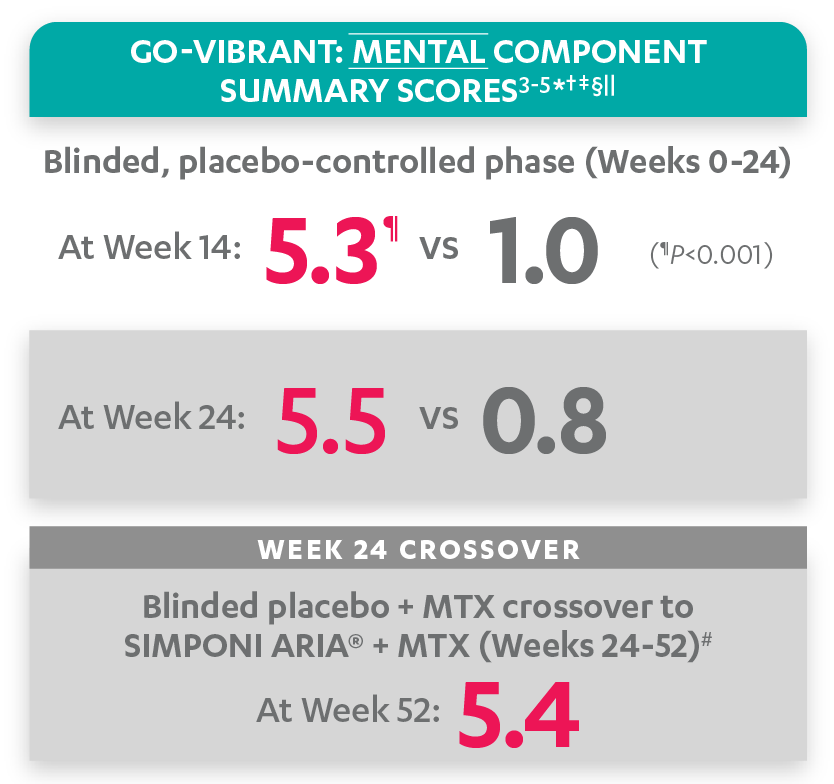
SF-36 MCS scores at Week 24 were not adjusted for multiplicity. Therefore, statistical significance has not been established.
In the GO-VIBRANT study, a change from baseline in SF-36 score, including PCS and MCS, of ≥5 points was considered clinically meaningful.
* Mean change from baseline in PCS scores and MCS scores is based on imputed data using last observation carried forward for missing data.
† No missing data imputation rule is applied.
‡ The P value is based on mixed-effect repeated measures model with treatment group, baseline MTX usage (Yes, No), baseline SF-36 PCS score, visit week, and an interaction of treatment and visit week as the terms in the model.
§ PCS and MCS scores at Week 14 were controlled endpoints that were tested sequentially among a list of other controlled endpoints only if the primary and major secondary endpoints achieved statistical significance.
|| SF-36 is a validated questionnaire. The same patients may not have responded at each timepoint.
# After Week 24, selected sponsor personnel were unblinded to subject-level data, which may have affected results.
The 8 domains of the SF-36 instrument are4:
Physical health domains
Mental health domains
Study design: GO-VIBRANT was a global, multicenter, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of SIMPONI ARIA® compared with placebo in 480 adult patients with active PsA. The target study population was biologic-naïve patients with active PsA for ≥6 months who met ClASsification criteria for Psoriatic ARthritis (CASPAR) criteria at screening. Patients in this trial had a diagnosis of PsA for at least 6 months and had symptoms of active disease (≥5 swollen joints and ≥5 tender joints and a CRP level of ≥0.6 mg/dL). At Week 0, patients were randomized in a 1:1 ratio to 1 of 2 treatment groups. Patients were allowed to be treated with or without MTX. Patients in the placebo group (n=239) were randomized to receive IV placebo infusions at Weeks 0, 4, 12, and 20. Patients in the SIMPONI ARIA® group (n=241) were randomized to receive SIMPONI ARIA® 2 mg/kg infusions at Weeks 0, 4, and q8w thereafter through Week 52. Patients were to receive a placebo infusion at Week 24 to maintain the treatment blind. At Week 24, all patients switched to treatment with SIMPONI ARIA® 2 mg/kg and were to receive administrations at Weeks 24, 28, and q8w through Week 52. The primary endpoint was the percentage of patients achieving an ACR20 response at Week 14.4
ACR20=20% improvement in American College of Rheumatology criteria; CRP=C-reactive protein; IV=intravenous; MTX=methotrexate; PsA=psoriatic arthritis; q8w=every 8 weeks; SF-36=36-item short-form survey; TNFα=tumor necrosis factor alpha.
NEXT: View FACIT-F in PsA »

PATIENT-REPORTED OUTCOMES (PROs) SIMPONI ARIA®
improvement in fatigue data as measured by FACIT-F in a clinical trial.

DOSING CALCULATOR
DOSING
CALCULATOR
Calculate an adult patient's dose of SIMPONI ARIA® based on weight.

References: 1. SIMPONI ARIA® (golimumab) [Prescribing Information]. Horsham, PA: Johnson & Johnson. 2. Humira® (adalimumab) [Prescribing Information]. North Chicago, IL: AbbVie Inc. 3. Kavanaugh A, Husni ME, Harrison DD, et al. Safety and efficacy of intravenous golimumab in patients with active psoriatic arthritis: results through week twenty-four of the GO-VIBRANT study. Arthritis Rheumatol. 2017;69(11):2151-2161. doi: 10.1002/art.40226. 4. Data on file. Johnson & Johnson. 5. Husni ME, Kavanaugh A, Murphy F, et al. Efficacy and safety of intravenous golimumab through one year in patients with active psoriatic arthritis. Arthrit Care Res. 2006;45(6):885-889.