- For US Healthcare Providers
- Full Prescribing Information
FOR ADULTS WITH MODERATELY TO SEVERELY ACTIVE RA, IN COMBINATION WITH MTX
DEMONSTRATED SAFETY PROFILE
Adverse events (AEs) through Week 241,2 :
| SIMPONI ARIA® + MTX | Placebo (saline) + MTX | ||
|---|---|---|---|
| Number of patients, n* | 463 | 197 | |
| Median follow-up, weeks | 24.0 | 23.9 | |
| Patients with ≥1 AE, % (n) | 52.9 (245) | 49.2 (97) | |
| Patients with ≥1 serious AE, % (n) | 4.1 (19) | 2.0 (4) | |
| Discontinuation rate due to AEs, % (n) | 3.5 (16) | 0.5 (1) | |
| Patients with ≥1 infection, % (n) | 26.6 (123) | 24.4 (48) | |
| Patients with ≥1 serious infection, % (n) | 0.9 (4) | 0.0 (0) | |
| Most common AE (occurring in ≥5% of patients treated with SIMPONI ARIA® 2 mg/kg + MTX) | |||
| Upper respiratory tract infection, % (n) | 6.5 (30) | 7.6 (15) | |
*Patients may appear in more than one column.
Incidence per 100 patient-years for patients treated with SIMPONI ARIA® + MTX (n=395) vs placebo (saline) + MTX (n=197) in the placebo-controlled phase1,2†:
- Active tuberculosis (TB): 0.00 (0 events; 95% confidence interval (CI) [0.00, 1.67] vs 0.00 (0 events; 95% CI [0.00, 3.79])
- Opportunistic infections: 0.56 (1 event; 95% CI [0.01, 3.10]) vs 0.00 (0 events; 95% CI [0.00, 3.79])
- Total malignancies: 0.56 (1 event; 95% CI [0.01, 3.11]) vs 0.00 (0 events; 95% CI [0.00, 3.79])
†Total patient-years of follow-up for patients receiving golimumab + MTX were 180 for active TB and opportunistic infections and 179 for total malignancies. Total patient-years of follow-up for the placebo (saline) + MTX group were 79.
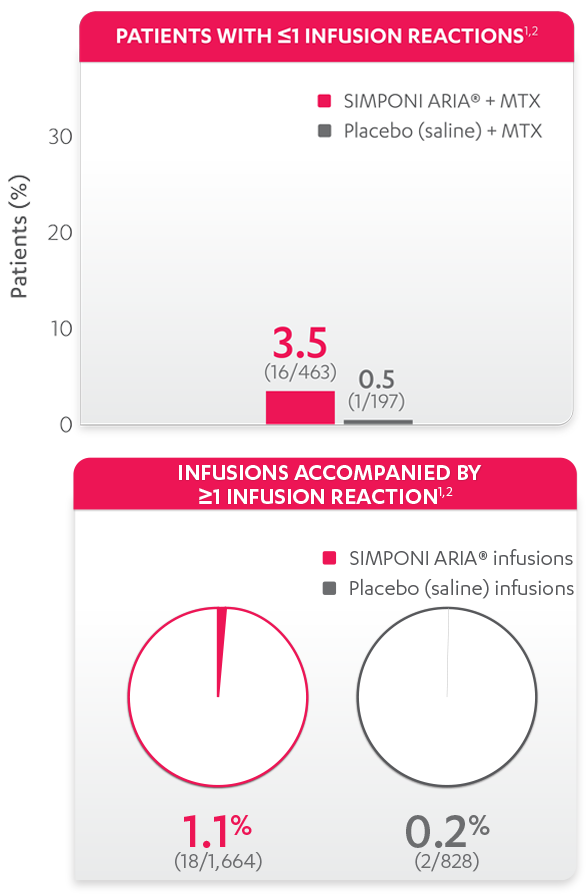
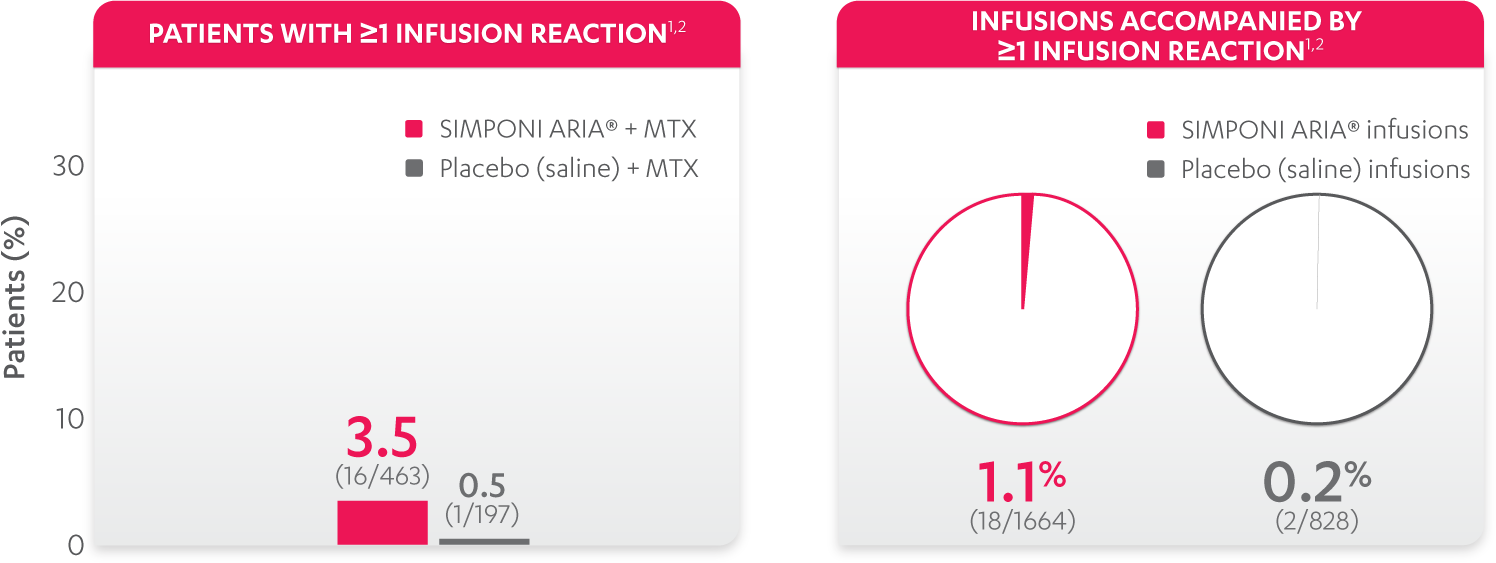
Infusion reactions reported through Week 241: