- For US Healthcare Providers
- Full Prescribing Information
FOR ADULTS WITH ACTIVE AS
DEMONSTRATED SAFETY PROFILE AT WEEK 60
Adverse events (AEs) through Week 601,2
Adverse events (AEs) through Week 601,2
| SIMPONI ARIA® | |
|---|---|
| Number of patients, n* | 204 |
| Median follow-up, weeks | 51.8 |
| Patients with ≥1 AE, % (n) | 55.4% (113) |
| Patients with ≥1 serious AE, % (n) | 3.4% (7) |
| Discontinuation rate due to AEs, % (n) | 2.0% (4) |
| Patients with ≥1 infection, % (n) | 32.8% (67) |
| Patients with ≥1 serious infection, % (n) | 1.5% (3) |
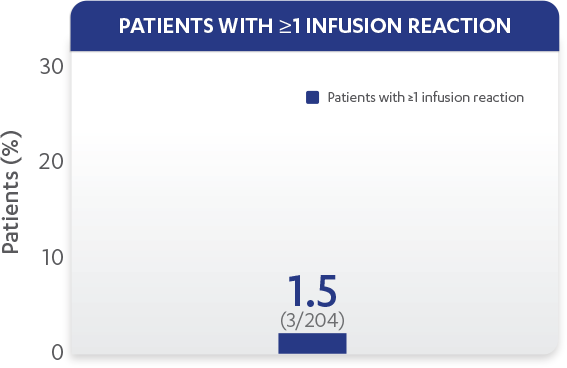
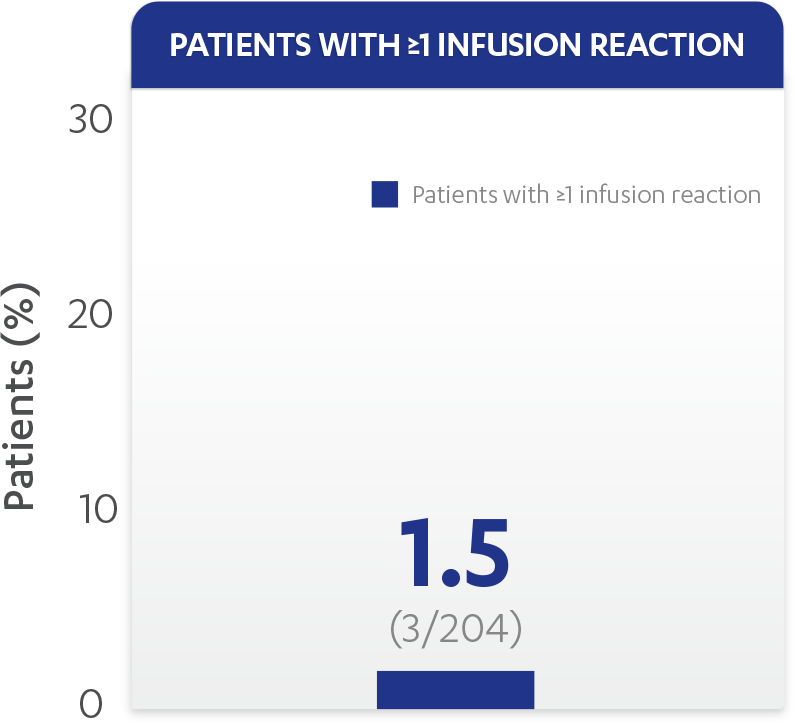
| Patients with ≥1 infusion reaction, % (n) | 1.5% (3) |
|
Most common AEs (occurring in ≥5% of patients treated with SIMPONI ARIA®)
Nasopharyngitis, 11.8% (24) |
|
*Patients may appear in more than one column.
- 1 case of active tuberculosis was reported
- No opportunistic infections or malignancies were reported
- No deaths were reported
Infusion reactions reported through Week 601,2