- For US Healthcare Providers
- Full Prescribing Information
FOR ADULTS WITH ACTIVE PsA
INHIBITION OF RADIOGRAPHIC PROGRESSION
ACR20 Response at Week 14 (primary endpoint): 75% of patients receiving SIMPONI ARIA® +/- MTX achieved ACR20 response vs 22% of patients receiving placebo +/- MTX (P<0.001)1-3
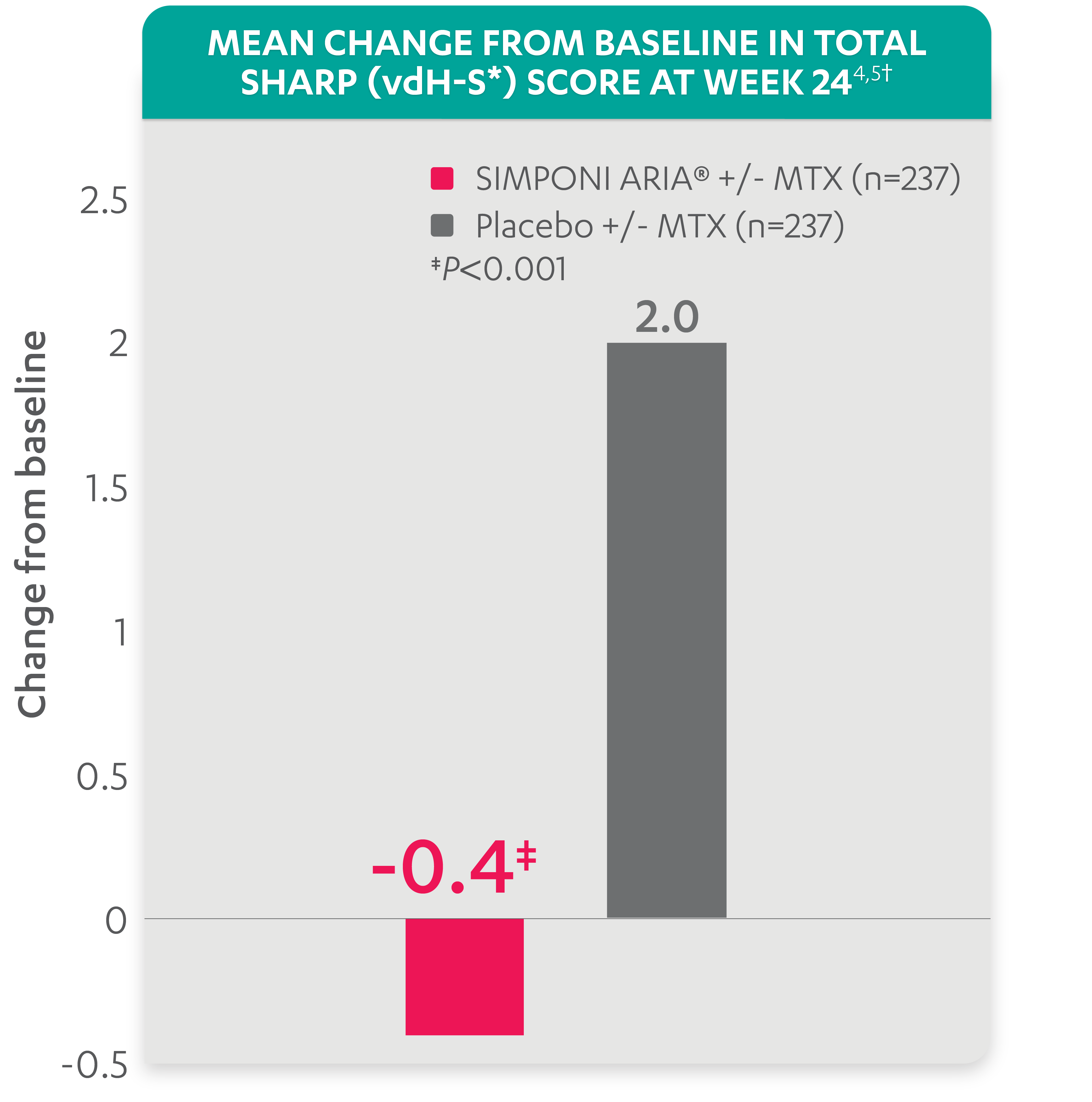
At Week 24: The mean change from baseline in total Sharp (vdH-S*) score†
-0.4
for patients receiving SIMPONI ARIA® +/- MTX (n=237) (P<0.001)
VS
2.0
for patients receiving placebo +/- MTX (n=237)1
A greater proportion of patients had
no progression of structural damage with SIMPONI ARIA®§
A greater proportion of patients had
no progression of structural damage
with SIMPONI ARIA®§
- At Week 24, 72% of SIMPONI ARIA® patients had no progression of structural damage compared with 43% of patients in the placebo group1‡
*vdH-S=van der Heijde Modified Sharp score. The total modified vdH-S score (0-528) is a composite score of structural damage that measures the number and size of joint erosions and the degree of joint space narrowing in the hands and feet.
†Change from baseline in total modified vdH-S score is based on imputed data using multiple imputation for missing data using predictive mean matching method.
§"No progression of structural damage” defined as change in the total modified vdH-S score ≤0.
Study design: GO-VIBRANT was a global, multicenter, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of SIMPONI ARIA® compared with placebo in 480 adult patients with active PsA. The target study population was biologic-naïve patients with active PsA for ≥6 months who met ClASsification criteria for Psoriatic ARthritis (CASPAR) criteria at screening. Patients in this trial had a diagnosis of PsA for at least 6 months and had symptoms of active disease (≥5 swollen joints and ≥5 tender joints and a CRP level of ≥0.6 mg/dL). At Week 0, patients were randomized in a 1:1 ratio to 1 of 2 treatment groups. Patients were allowed to be treated with or without MTX. Patients in the placebo group (n=239) were randomized to receive IV placebo infusions at Weeks 0, 4, 12, and 20. Patients in the SIMPONI ARIA® group (n=241) were randomized to receive SIMPONI ARIA®2 mg/kg infusions at Weeks 0, 4, and q8w thereafter through Week 52. Patients were to receive a placebo infusion at Week 24 to maintain the treatment blind. At Week 24, all patients switched to treatment with SIMPONI ARIA®2 mg/kg and were to receive administrations at Weeks 24, 28, and q8w through Week 52. The primary endpoint was the percentage of patients achieving an ACR20 response at Week 14.