- For US Healthcare Providers
- Full Prescribing Information
FOR ADULTS WITH ACTIVE PsA
SIMPONI ARIA® IS THE FIRST AND ONLY FULLY HUMAN ANTI-TNFα BIOLOGIC WITH IMPROVEMENT IN BOTH PHYSICAL AND MENTAL COMPONENT SUMMARY SCORES OF SF-36 IN THE LABEL
ACR20 Response at Week 14 (primary endpoint): 75% of patients receiving SIMPONI ARIA® +/- MTX achieved ACR20 response vs 22% of patients receiving placebo +/- MTX (P<0.001)1-3
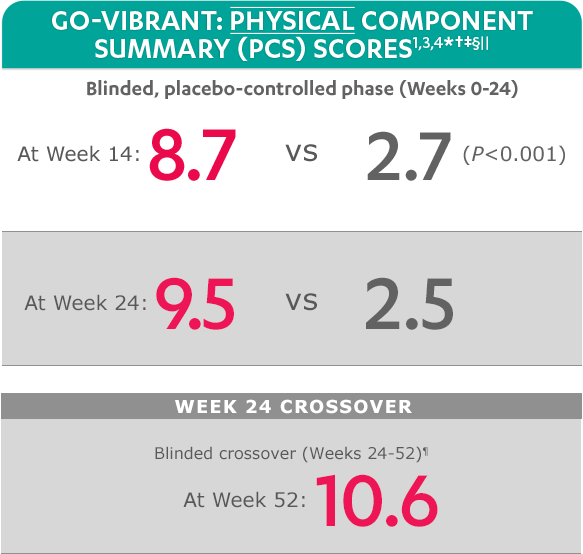
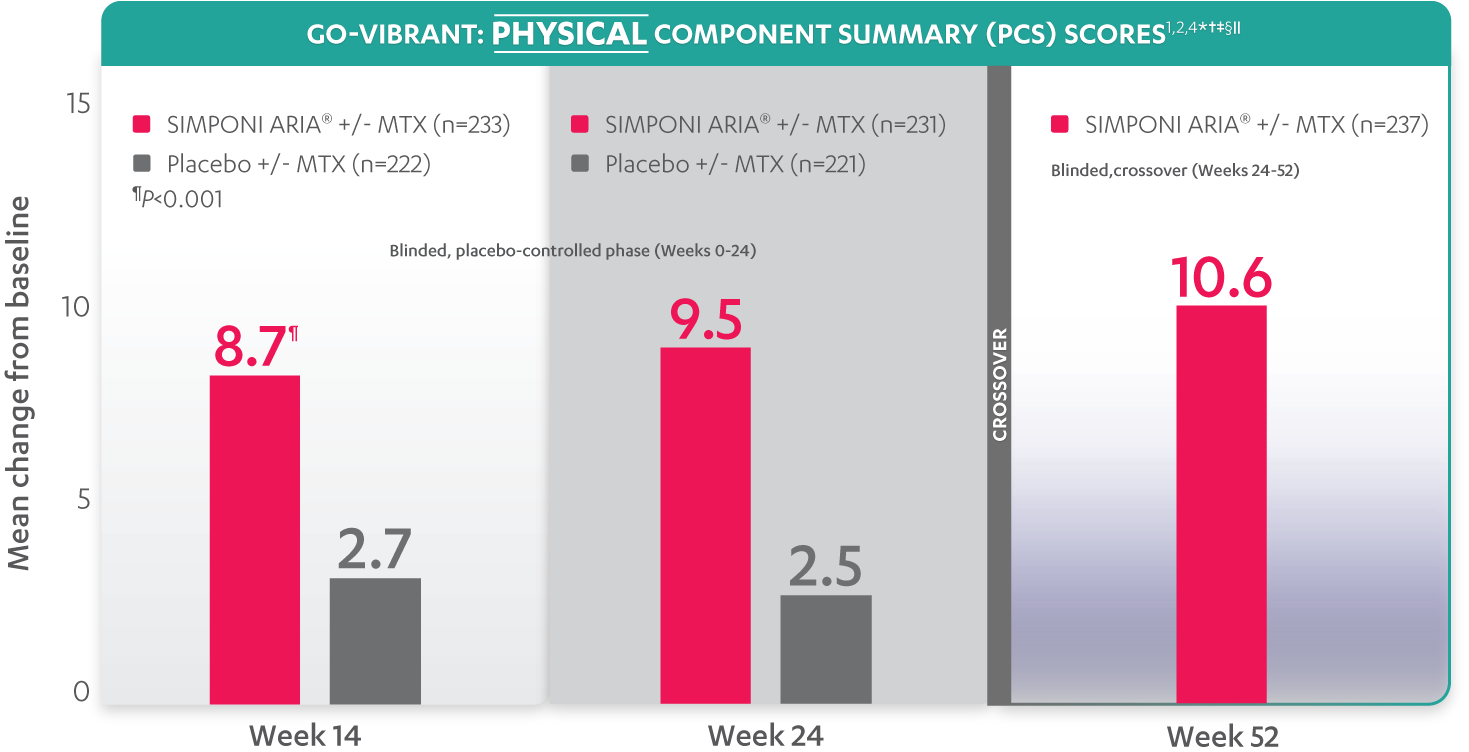
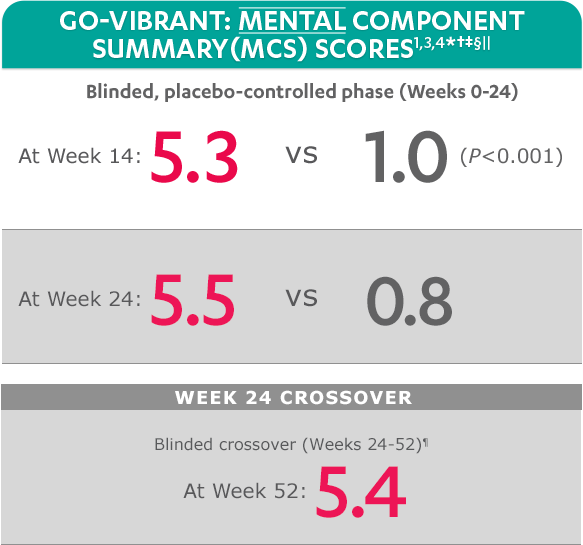
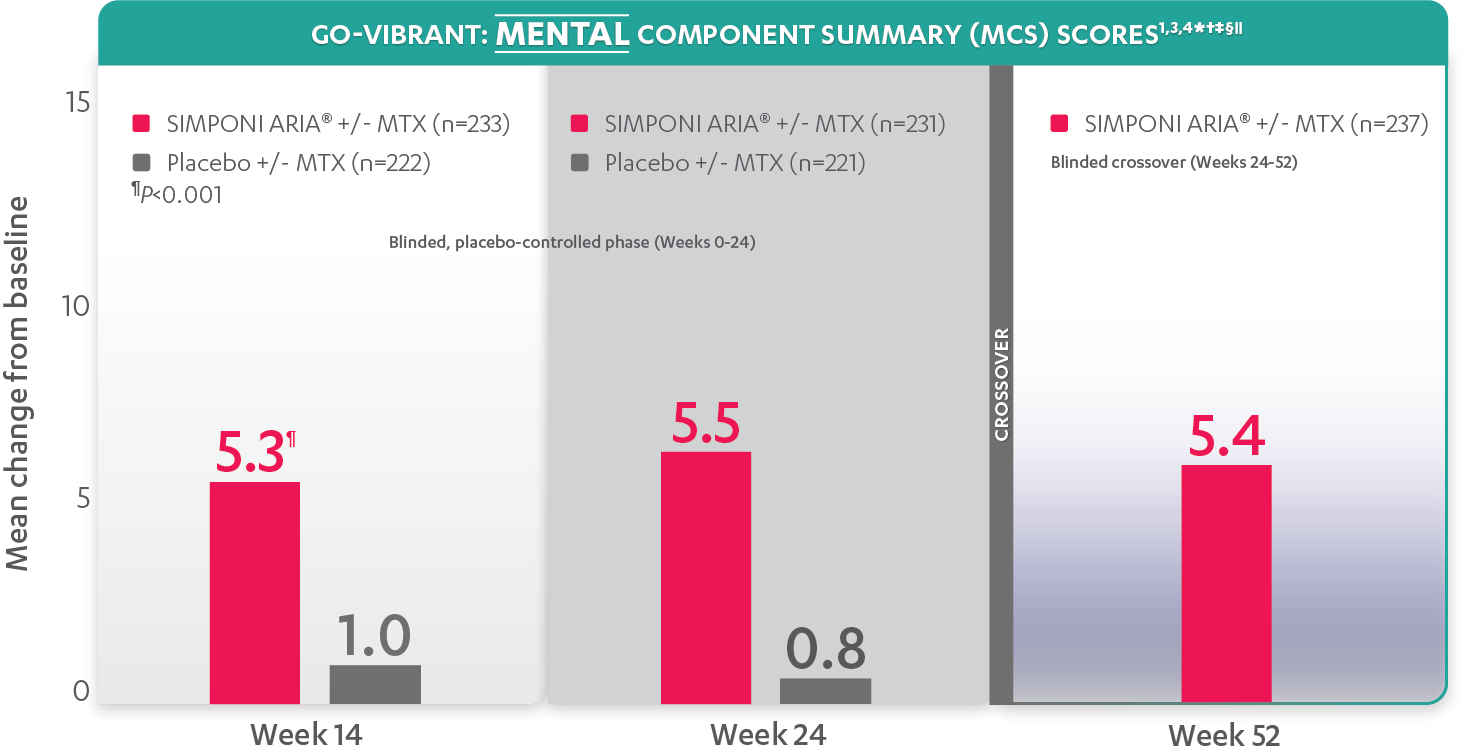
SF-36 is a 36-item short-form health survey for patients. This instrument yields an 8-domain profile of functional health and well-being scores, as well as psychometrically based physical component summary (PCS) and mental component summary (MCS) scores.
Mean change from baseline for patients receiving SIMPONI ARIA® +/- MTX (n=233) vs patients receiving placebo +/- MTX (n=222)
SF-36 PCS scores at Week 24 were not adjusted for multiplicity. Therefore, statistical significance has not been established.
In the GO-VIBRANT study, a change from baseline in SF-36 score, including PCS and MCS, of ≥5 points was considered clinically meaningful.
Mean change from baseline for patients receiving SIMPONI ARIA® +/- MTX (n=233) vs patients receiving placebo +/- MTX (n=222)
SF-36 MCS scores at Week 24 were not adjusted for multiplicity. Therefore, statistical significance has not been established.
In the GO-VIBRANT study, a change from baseline in SF-36 score, including PCS and MCS, of ≥5 points was considered clinically meaningful.
*Mean change from baseline in PCS scores and MCS scores is based on imputed data using LOCF for missing data.
†No missing data imputation rule is applied.
‡The P value is based on Mixed-Effect Repeated Measures (MMRM) model with treatment group, baseline MTX usage (Yes, No), baseline SF-36 PCS score, visit week, and an interaction of treatment and visit week as the terms in the model.
§PCS and MCS scores at Week 14 were controlled endpoints that were tested sequentially among a list of other controlled endpoints only if the primary and major secondary endpoints achieved statistical significance.
||SF-36 is a validated questionnaire. The same patients may not have responded at each timepoint.
¶After Week 24, patients and doctors knew that all patients were on SIMPONI ARIA® (blinded active treatment), which may have affected the results.
The 8 multi-item domains of the SF-36 instrument are1:
Physical health domains
- Limitations in physical functioning due to health problems
- Limitations in usual role activities due to physical health problems
- Bodily pain
- General health perception
Mental health domains
- Limitations in social functioning due to physical or mental health problems
- Limitations in usual role activities due to personal or emotional problems
- Vitality (energy and fatigue)
- General mental health (psychological distress and well-being)
Study design: GO-VIBRANT was a global, multicenter, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of SIMPONI ARIA® compared with placebo in 480 adult patients with active PsA. The target study population was biologic-naïve patients with active PsA for ≥6 months who met ClASsification criteria for Psoriatic ARthritis (CASPAR) criteria at screening. Patients in this trial had a diagnosis of PsA for at least 6 months and had symptoms of active disease (≥5 swollen joints and ≥5 tender joints and a CRP level of ≥0.6 mg/dL). At Week 0, patients were randomized in a 1:1 ratio to 1 of 2 treatment groups. Patients were allowed to be treated with or without MTX. Patients in the placebo group (n=239) were randomized to receive IV placebo infusions at Weeks 0, 4, 12, and 20. Patients in the SIMPONI ARIA® group (n=241) were randomized to receive SIMPONI ARIA® 2-mg/kg infusions at Weeks 0, 4, and q8w thereafter through Week 52. Patients were to receive a placebo infusion at Week 24 to maintain the treatment blind. At Week 24, all patients switched to treatment with SIMPONI ARIA® 2 mg/kg and were to receive administrations at Weeks 24, 28, and q8w through Week 52. The primary endpoint was the percentage of patients achieving an ACR20 response at Week 14.
PATIENT-REPORTED OUTCOMES (PROs)
SIMPONI ARIA® improvement in fatigue data as measured by FACIT-F in a clinical trial.
DOSING CALCULATOR
Calculate an adult patient's dose of SIMPONI ARIA® based on weight.
ACCESS AND AFFORDABILITY
View first-line coverage rates for SIMPONI ARIA®.