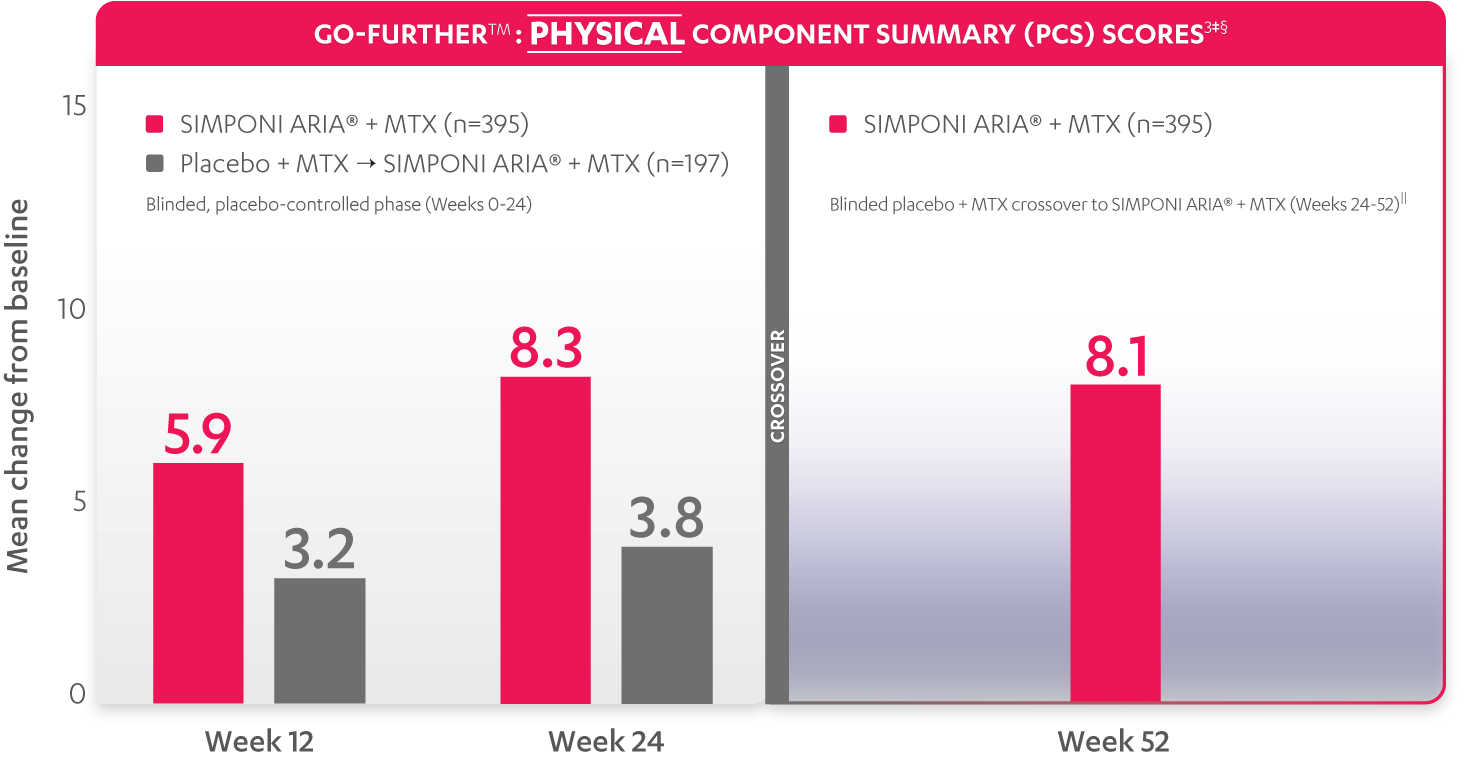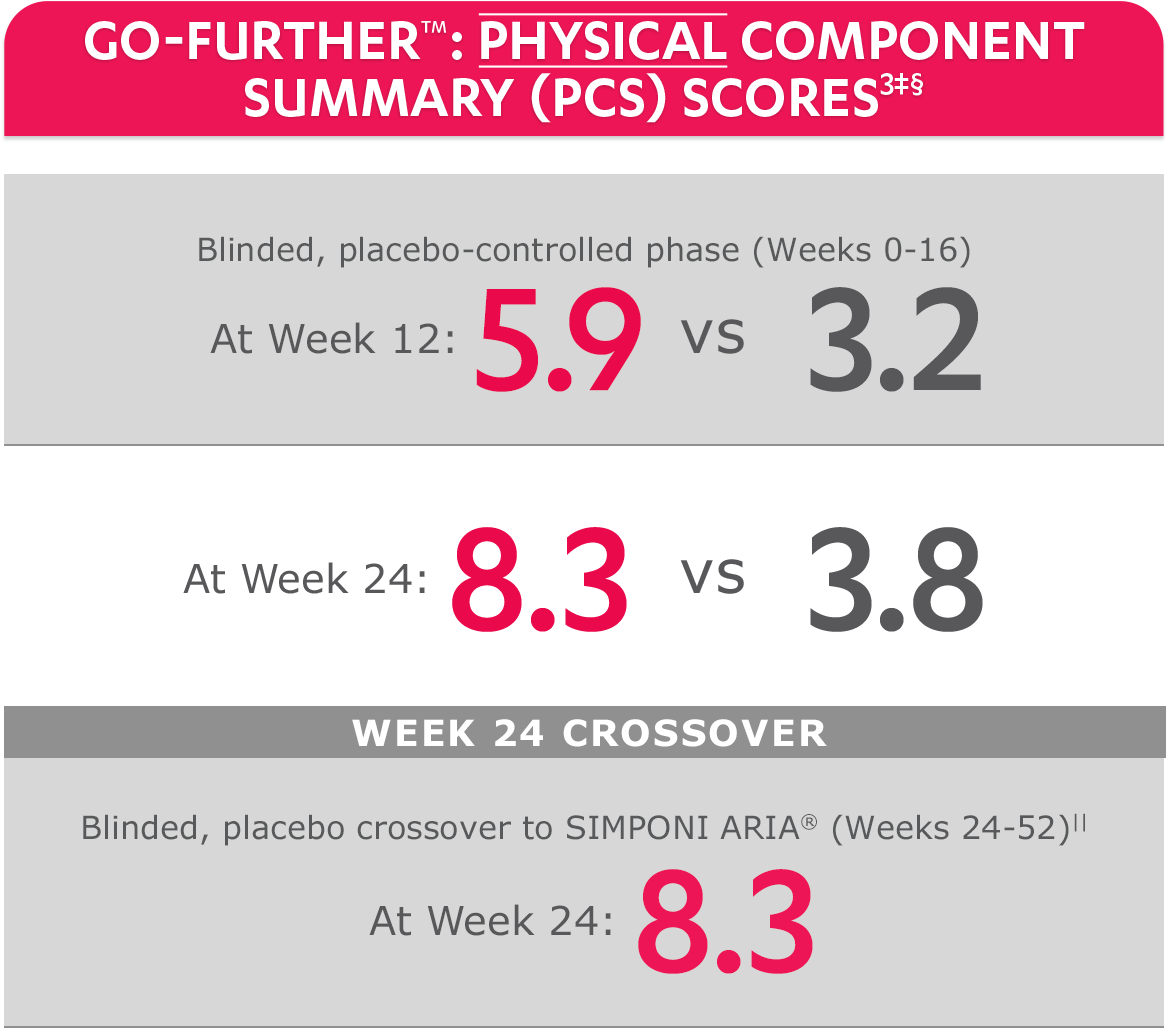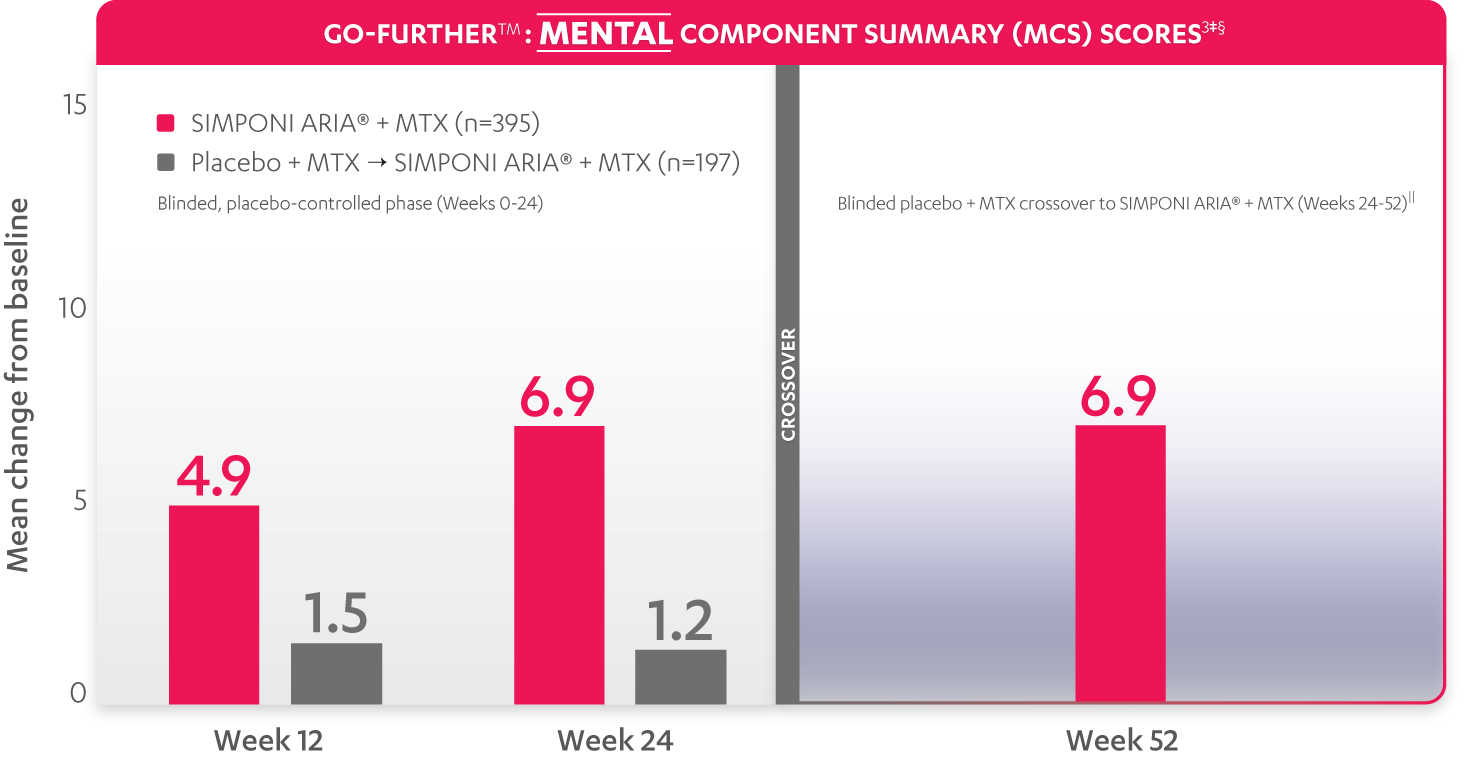
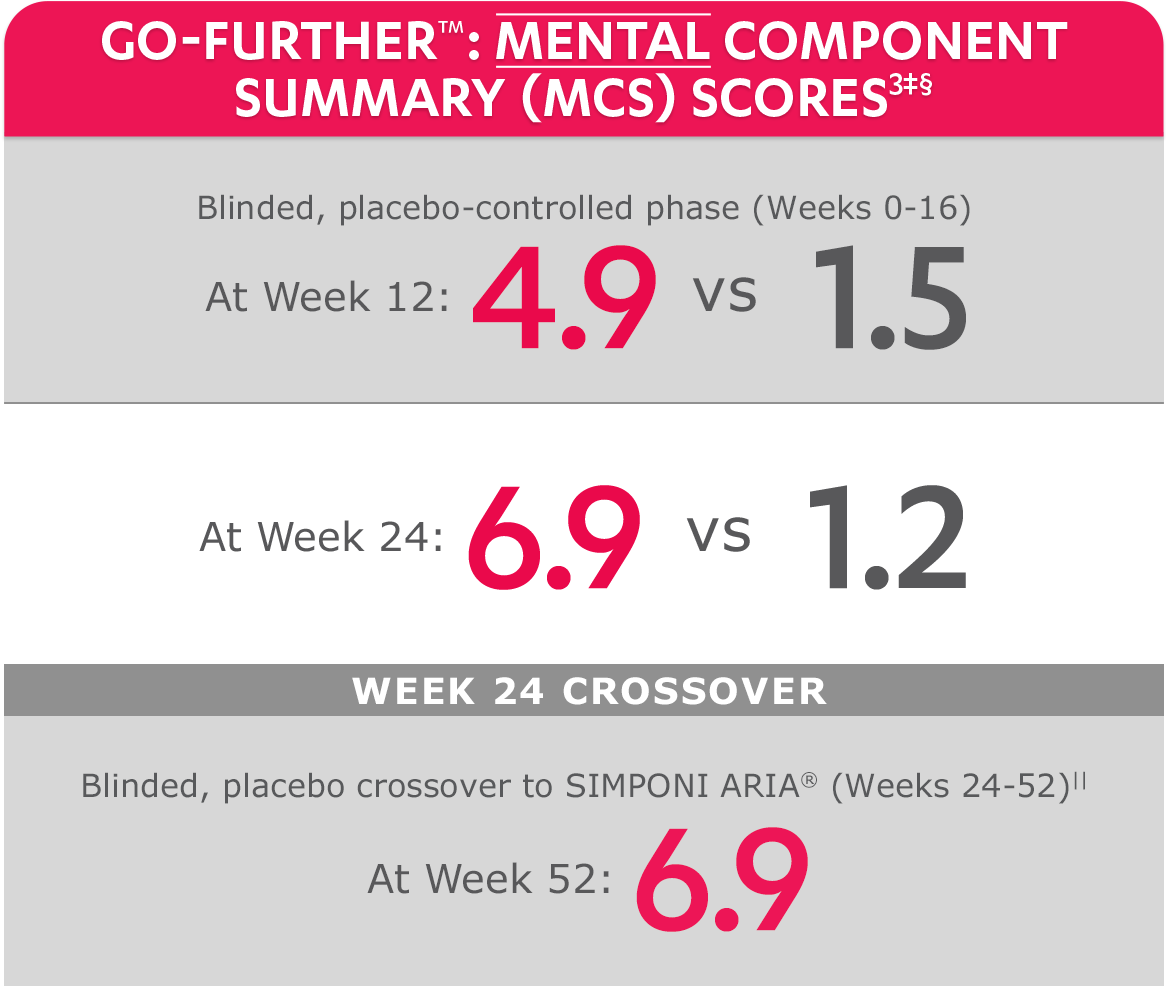
SF-36 MCS scores were not adjusted for multiplicity. Therefore, statistical significance has not been established.
In the GO-FURTHER™ study, a change from baseline in SF-36 score, including PCS and MCS, of ≥5 points was considered clinically meaningful.
‡The randomized (intent-to-treat) population was used for these analyses.
§SF-36 is a validated questionnaire. The same patients may not have responded at each time point. Mean change from baseline in SF-36 score is based on imputed data using LOCF for missing data.
ǁAfter Week 24, patients and doctors knew that all patients were on SIMPONI ARIA® (blinded active treatment), which may have affected the results.