- For US Healthcare Providers
- Full Prescribing Information
FOR ADULTS WITH MODERATELY TO SEVERELY ACTIVE RA, IN COMBINATION WITH MTX
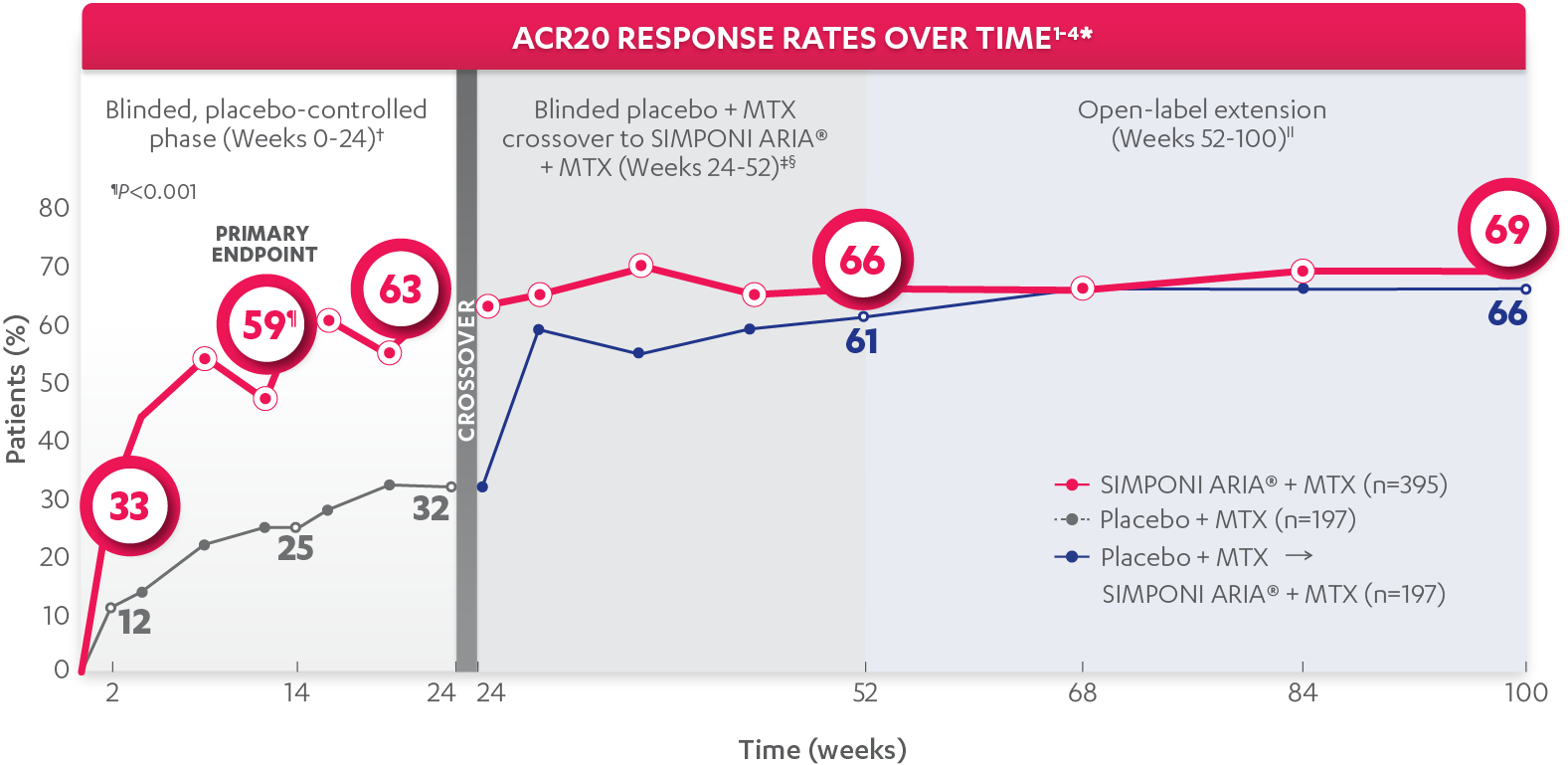
DEMONSTRATED SYMPTOM CONTROL IN MODERATELY TO SEVERELY ACTIVE RA
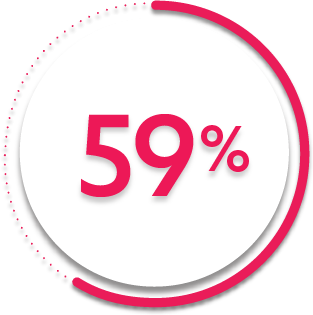
ACR20 response at Week 14 (primary endpoint):
59%
of patients receiving SIMPONI ARIA® + MTX (231/395) achieved ACR20 response (P<0.001)
VS
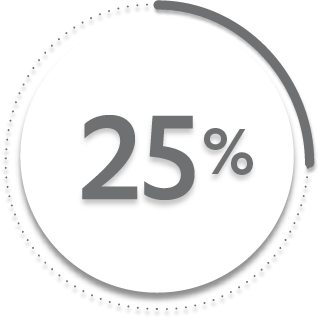
25%
of patients receiving placebo + MTX (49/197)1-3
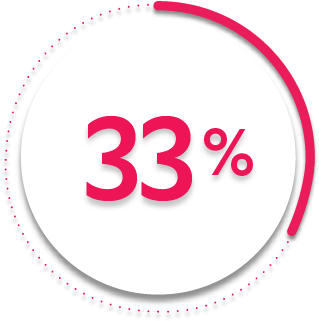
Rapid ACR20 response at Week 2:
33%
of patients receiving SIMPONI ARIA® + MTX (131/395) achieved ACR20 response
VS
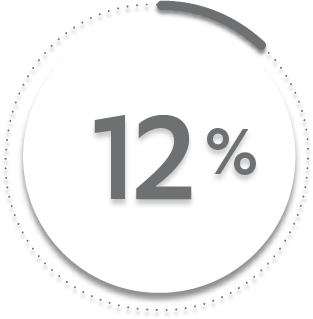
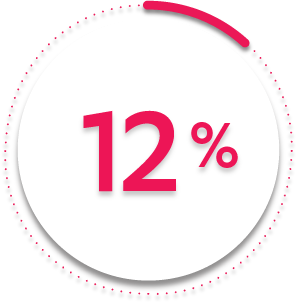
12%
of patients receiving placebo + MTX (23/197)1-3
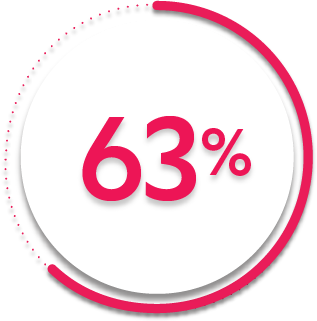
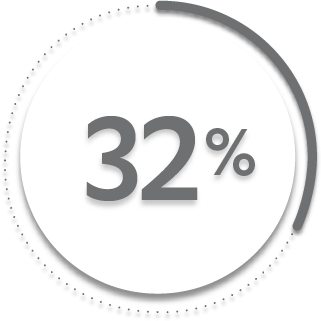
ACR20 response at Week 24:
63%
of patients receiving SIMPONI ARIA® + MTX (248/395) achieved ACR20 response
VS
32%
of patients receiving placebo + MTX (63/197)1-3
ACR20 responses at Week 2 and Week 24 were not adjusted for multiplicity. Therefore, statistical significance has not been established.
ACR20 response at Week 52§:
66%
of patients receiving SIMPONI ARIA® + MTX (260/395) achieved ACR20 response
VS
61%
of patients receiving placebo who crossed over to SIMPONI ARIA® + MTX at Week 24 (121/197)2
ACR20 response at Week 100||:
69%
of patients receiving SIMPONI ARIA® + MTX (273/395) achieved ACR20 response
VS
66%
of patients receiving placebo who crossed over to SIMPONI ARIA® + MTX at Week 24 (130/197)4
ACR20 responses at Week 2 and Week 24 were not adjusted for multiplicity. Therefore, statistical significance has not been established.
*The same patients may not have responded at each time point.
†In this intention-to-treat (ITT) analysis, patients were considered to be nonresponders if they experienced any of the following: increased MTX dose above baseline for RA; initiated treatment with DMARDs (including commercial MTX), systemic immunosuppressives, and/or biologics for RA; initiated treatment with oral corticosteroids for RA above baseline dose or received intravenous (IV) or intramuscular corticosteroids for RA; or discontinued treatment due to an unsatisfactory therapeutic effect. Last observation carried forward (LOCF) rules were applied to missing data. The patients who early escaped at Week 16 also had Week 16 data carried forward up through Week 24.
‡At Week 24, all remaining patients in the placebo + MTX group began receiving SIMPONI ARIA® + MTX in a blinded manner.
§In this ITT analysis, all rules were nullified and reapplied after Week 24. Patients were considered to be nonresponders if they discontinued treatment due to an unsatisfactory therapeutic effect.
||At Week 52, all study personnel were unblinded to subject-level data.
Study design: GO-FURTHER™ was a global, multicenter, randomized, double-blind, placebo-controlled study in 592 adult patients who had moderately to severely active RA despite a stable dose of MTX (15-25 mg/week) for ≥3 months and who had not been previously treated with an anti-TNF agent. Moderately to severely active RA was defined as ≥6 swollen joints (out of 66 total) and ≥6 tender joints (out of 68 total), RF positive and/or anti-CCP antibody positive, and CRP ≥1.0 mg/dL. Patients were randomized to receive SIMPONI ARIA® 2 mg/kg + MTX (n=395) or placebo + MTX (n=197) as a 30-minute IV infusion at Weeks 0 and 4, and then q8 weeks through Week 100. At Week 16, patients in the placebo + MTX group with <10% improvement from baseline in both swollen joint count and tender joint count began receiving SIMPONI ARIA® 2 mg/kg beginning with an induction regimen at Weeks 16 and 20, followed by maintenance infusions q8 weeks in a blinded manner. At Week 24, all patients remaining in the placebo + MTX group began receiving SIMPONI ARIA® 2 mg/kg beginning with an induction regimen at Weeks 24 and 28, followed by maintenance infusions q8 weeks in a blinded manner. All patients continued to receive MTX. The primary endpoint was the percentage of patients achieving an ACR20 response at Week 14.
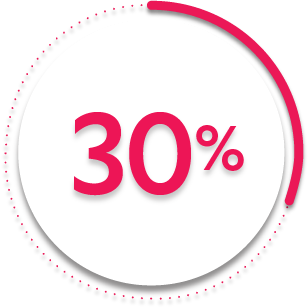
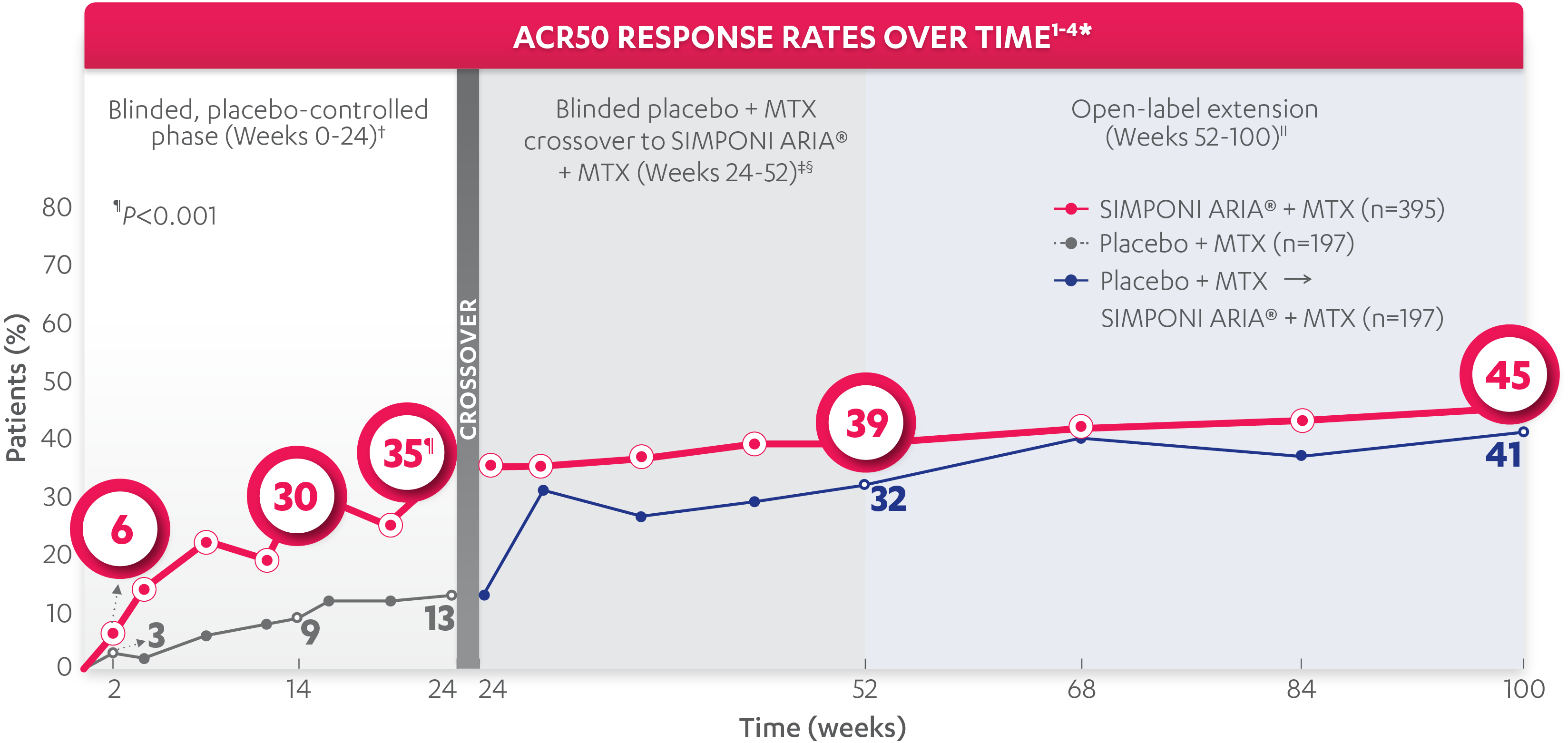
ACR50 response at Week 14:
30%
of patients receiving SIMPONI ARIA® + MTX (118/395) achieved ACR50 response
VS
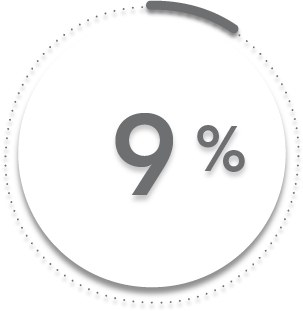
9%
of patients receiving placebo + MTX (17/197)1-3
ACR50 response at Week 24:
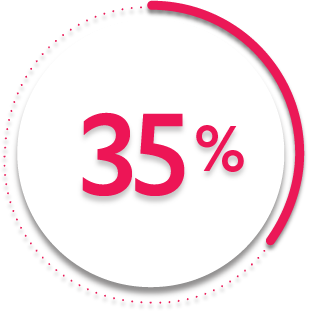
35%
of patients receiving SIMPONI ARIA® + MTX (138/395) achieved ACR50 response (P<0.001)
VS
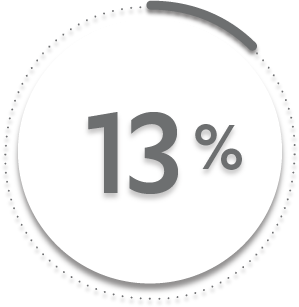
13%
of patients receiving placebo + MTX (26/197)1-3
ACR50 responses at Week 2 and Week 14 were not adjusted for multiplicity. Therefore, statistical significance has not been established.
ACR50 response at Week 52§:
39%
of patients receiving SIMPONI ARIA® + MTX (153/395) achieved ACR50 response
VS
32%
of patients receiving placebo who crossed over to SIMPONI ARIA® + MTX at Week 24 (62/197)2
ACR50 response at Week 100||:
45%
of patients receiving SIMPONI ARIA® + MTX (178/395) achieved ACR50 response
VS
41%
of patients receiving placebo who crossed over to SIMPONI ARIA® + MTX at Week 24 (81/197)4
*The same patients may not have responded at each time point.
†In this intention-to-treat (ITT) analysis, patients were considered to be nonresponders if they experienced any of the following: increased MTX dose above baseline for RA; initiated treatment with DMARDs (including commercial MTX), systemic immunosuppressives, and/or biologics for RA; initiated treatment with oral corticosteroids for RA above baseline dose or received intravenous (IV) or intramuscular corticosteroids for RA; or discontinued treatment due to an unsatisfactory therapeutic effect. Last observation carried forward (LOCF) rules were applied to missing data. The patients who early escaped at Week 16 also had Week 16 data carried forward up through Week 24.
‡At Week 24, all remaining patients in the placebo + MTX group began receiving SIMPONI ARIA® + MTX in a blinded manner.
§In this ITT analysis, all rules were nullified and reapplied after Week 24. Patients were considered to be nonresponders if they discontinued treatment due to an unsatisfactory therapeutic effect.
||At Week 52, all study personnel were unblinded to subject-level data.
Study design: GO-FURTHER™ was a global, multicenter, randomized, double-blind, placebo-controlled study in 592 adult patients who had moderately to severely active RA despite a stable dose of MTX (15-25 mg/week) for ≥3 months and who had not been previously treated with an anti-TNF agent. Moderately to severely active RA was defined as ≥6 swollen joints (out of 66 total) and ≥6 tender joints (out of 68 total), RF positive and/or anti-CCP antibody positive, and CRP ≥1.0 mg/dL. Patients were randomized to receive SIMPONI ARIA® 2 mg/kg + MTX (n=395) or placebo + MTX (n=197) as a 30-minute IV infusion at Weeks 0 and 4, and then q8 weeks through Week 100. At Week 16, patients in the placebo + MTX group with <10% improvement from baseline in both swollen joint count and tender joint count began receiving SIMPONI ARIA® 2 mg/kg beginning with an induction regimen at Weeks 16 and 20, followed by maintenance infusions q8 weeks in a blinded manner. At Week 24, all patients remaining in the placebo + MTX group began receiving SIMPONI ARIA® 2 mg/kg beginning with an induction regimen at Weeks 24 and 28, followed by maintenance infusions q8 weeks in a blinded manner. All patients continued to receive MTX. The primary endpoint was the percentage of patients achieving an ACR20 response at Week 14.
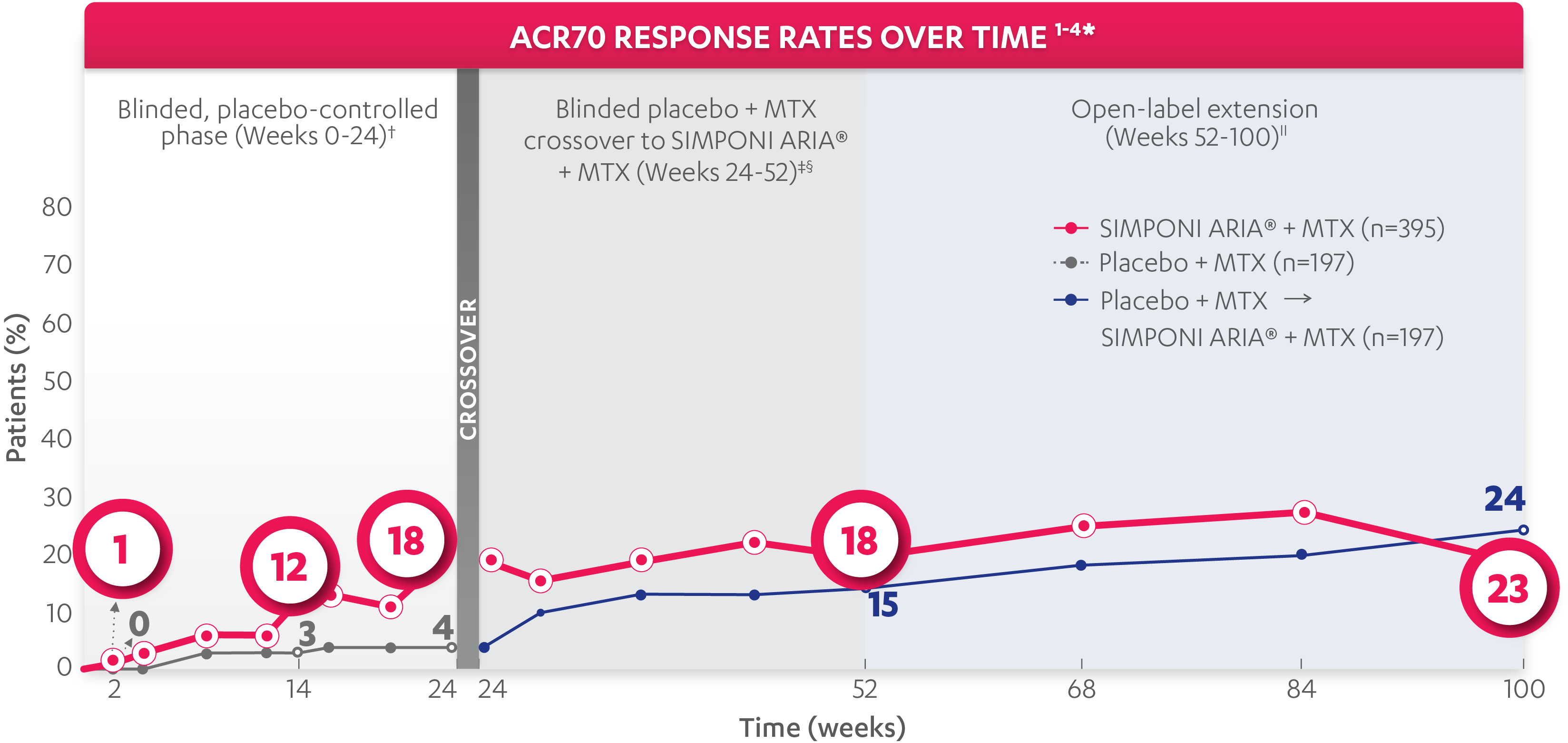
ACR70 response at Week 14:
12%
of patients receiving SIMPONI ARIA® + MTX (49/395) achieved ACR70 response
VS
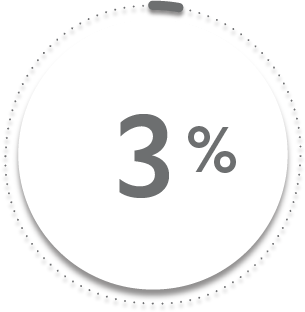
3%
of patients receiving placebo + MTX (6/197)1-3
ACR70 response at Week 24:
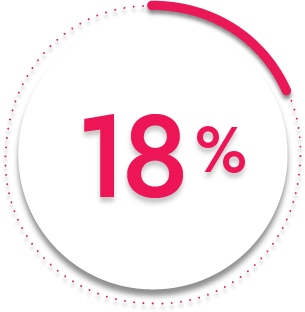
18%
of patients receiving SIMPONI ARIA® + MTX (69/395) achieved ACR70 response
VS
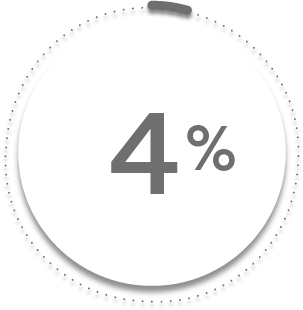
4%
of patients receiving placebo + MTX (8/197)1-3
ACR70 responses were not adjusted for multiplicity. Therefore, statistical significance has not been established.
ACR70 response at Week 52:
18%
of patients receiving SIMPONI ARIA® + MTX (72/395) achieved ACR70 response
VS
15%
of patients receiving placebo who crossed over to SIMPONI ARIA® + MTX at Week 24 (29/197)2
ACR70 response at Week 100||:
24%
of patients receiving SIMPONI ARIA® + MTX (95/395) achieved ACR70 response
VS
23%
of patients receiving placebo who crossed over to SIMPONI ARIA® + MTX at Week 24 (45/197)4
ACR70 responses were not adjusted for multiplicity. Therefore, statistical significance has not been established.
*The same patients may not have responded at each time point.
†In this intention-to-treat (ITT) analysis, patients were considered to be nonresponders if they experienced any of the following: increased MTX dose above baseline for RA; initiated treatment with DMARDs (including commercial MTX), systemic immunosuppressives, and/or biologics for RA; initiated treatment with oral corticosteroids for RA above baseline dose or received intravenous (IV) or intramuscular corticosteroids for RA; or discontinued treatment due to an unsatisfactory therapeutic effect. Last observation carried forward (LOCF) rules were applied to missing data. The patients who early escaped at Week 16 also had Week 16 data carried forward up through Week 24.
‡At Week 24, all remaining patients in the placebo + MTX group began receiving SIMPONI ARIA® + MTX in a blinded manner.
§In this ITT analysis, all rules were nullified and reapplied after Week 24. Patients were considered to be nonresponders if they discontinued treatment due to an unsatisfactory therapeutic effect.
||At Week 52, all study personnel were unblinded to subject-level data.
Study design: GO-FURTHER™ was a global, multicenter, randomized, double-blind, placebo-controlled study in 592 adult patients who had moderately to severely active RA despite a stable dose of MTX (15-25 mg/week) for ≥3 months and who had not been previously treated with an anti-TNF agent. Moderately to severely active RA was defined as ≥6 swollen joints (out of 66 total) and ≥6 tender joints (out of 68 total), RF positive and/or anti-CCP antibody positive, and CRP ≥1.0 mg/dL. Patients were randomized to receive SIMPONI ARIA® 2 mg/kg + MTX (n=395) or placebo + MTX (n=197) as a 30-minute IV infusion at Weeks 0 and 4, and then q8 weeks through Week 100. At Week 16, patients in the placebo + MTX group with <10% improvement from baseline in both swollen joint count and tender joint count began receiving SIMPONI ARIA® 2 mg/kg beginning with an induction regimen at Weeks 16 and 20, followed by maintenance infusions q8 weeks in a blinded manner. At Week 24, all patients remaining in the placebo + MTX group began receiving SIMPONI ARIA® 2 mg/kg beginning with an induction regimen at Weeks 24 and 28, followed by maintenance infusions q8 weeks in a blinded manner. All patients continued to receive MTX. The primary endpoint was the percentage of patients achieving an ACR20 response at Week 14.