For US Healthcare Providers
For US Healthcare Providers
FOR ADULTS WITH ACTIVE AS
DEMONSTRATED SAFETY PROFILE AT WEEK 60
Adverse events (AEs) through Week 601,2
Adverse events (AEs) through Week 601,2
| SIMPONI ARIA® | |
|---|---|
| Number of patients, n* | 204 |
| Median follow-up, weeks | 51.8 |
| Patients with ≥1 AE, % (n) | 55.4% (113) |
| Patients with ≥1 serious AE, % (n) | 3.4% (7) |
| Discontinuation rate due to AEs, % (n) | 2.0% (4) |
| Patients with ≥1 infection, % (n) | 32.8% (67) |
| Patients with ≥1 serious infection, % (n) | 1.5% (3) |
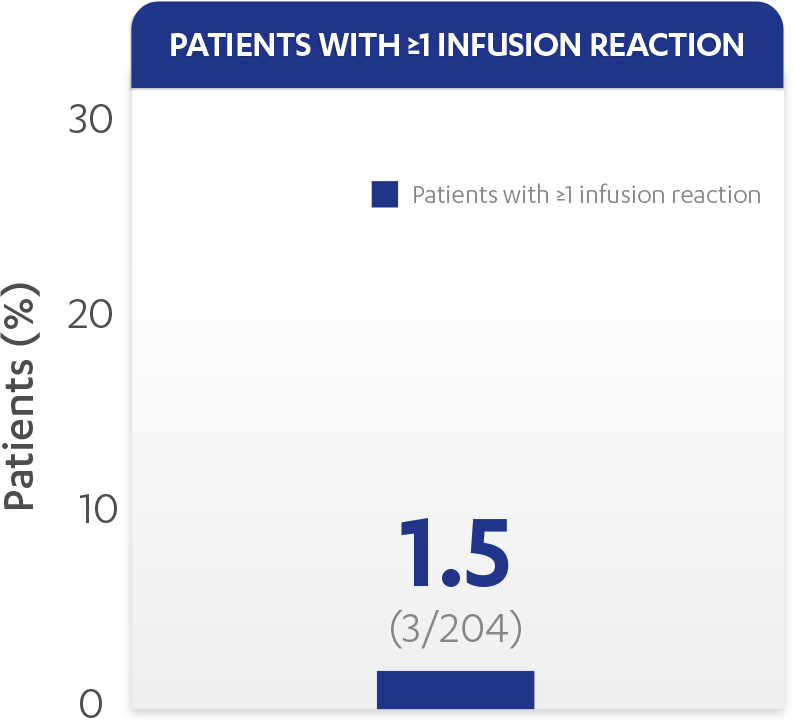
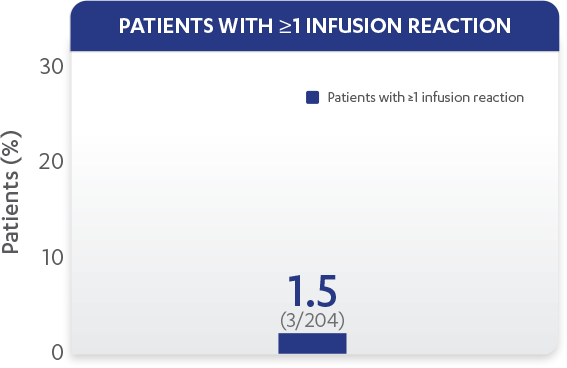
| Patients with ≥1 infusion reaction, % (n) | 1.5% (3) |
| Most common AEs (occurring in ≥5% of patients treated with SIMPONI ARIA®) | Nasopharyngitis, 11.8% (24) Upper respiratory tract infection, 7.4% (15) Alanine aminotransferase (ALT) increased, 5.9% (12) |
Most common AEs (occurring in ≥5% of patients treated with SIMPONI ARIA®) Nasopharyngitis, 11.8% (24) | |
*Patients may appear in more than one column.
- 1 case of active tuberculosis was reported
- No opportunistic infections or malignancies were reported
- No deaths were reported
Infusion reactions reported through Week 601,2