- For US Healthcare Providers
- Full Prescribing Information
FOR ADULTS WITH ACTIVE PsA
SKIN SYMPTOM RESPONSE
ACR20 Response at Week 14 (primary endpoint): 75% of patients receiving SIMPONI ARIA® +/- MTX achieved ACR20 response vs 22% of patients receiving placebo +/- MTX (P<0.001)1-3
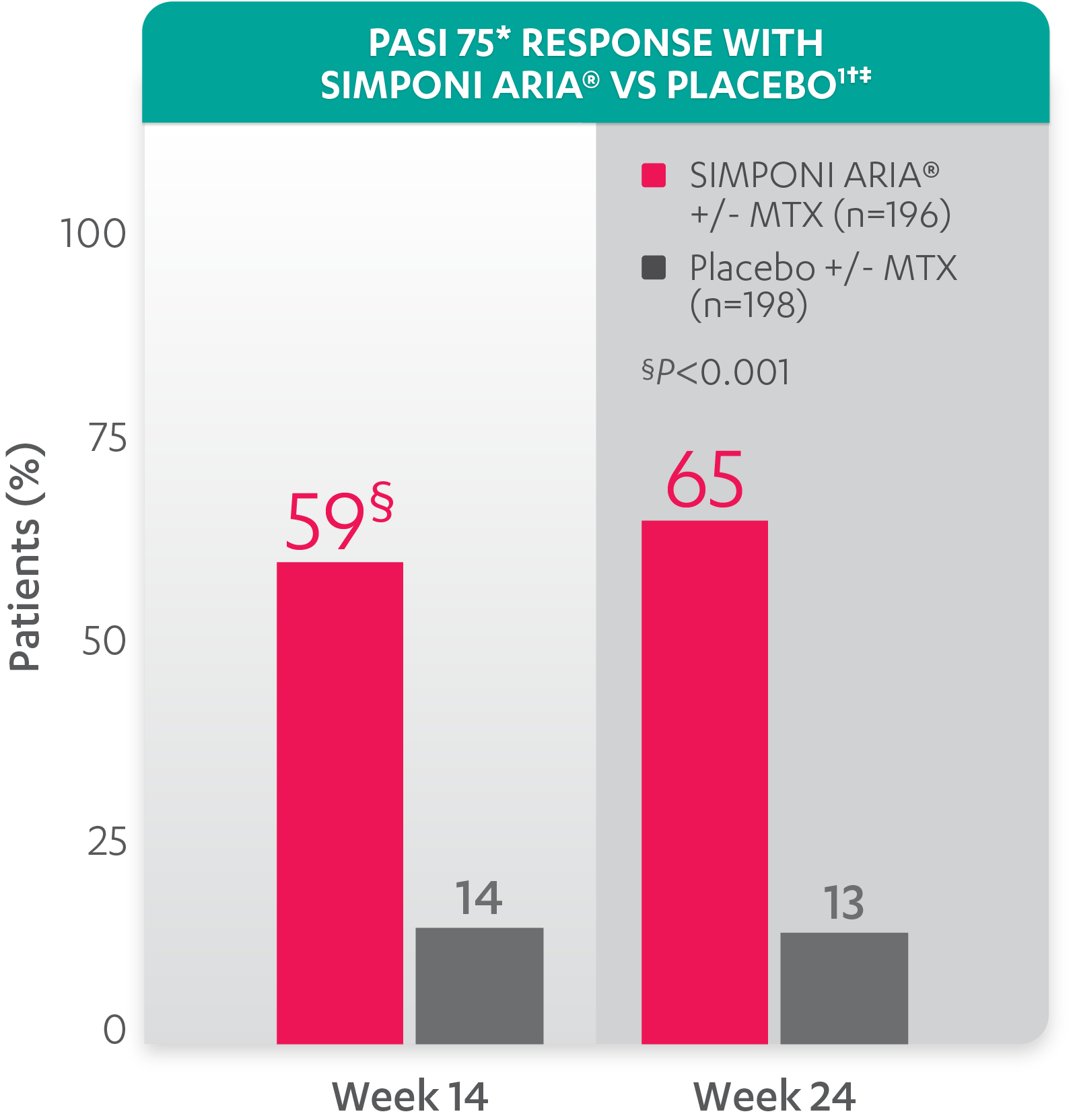
PASI response with SIMPONI ARIA® vs placebo
SIMPONI ARIA® has not been studied in and is not indicated for the treatment of plaque psoriasis. The GO-VIBRANT study population included patients with wide ranges of psoriasis disease severity not reflective of a plaque psoriasis population. These data do not provide evidence of a clinically meaningful improvement in mild to moderate psoriasis.
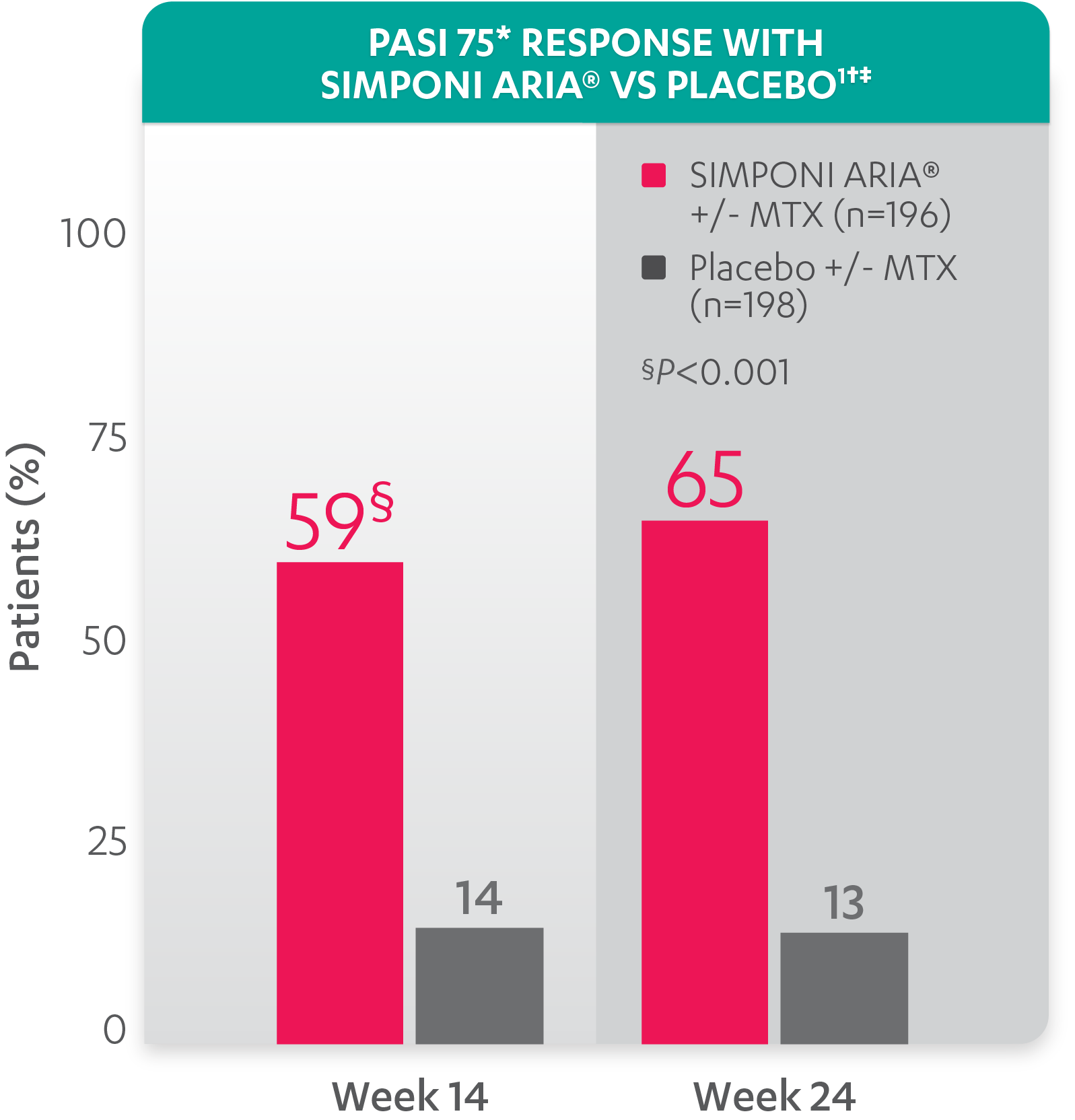
PASI 75 at Week 24 was not adjusted for multiplicity. Therefore, statistical significance has not been established.
*PASI=Psoriasis Area and Severity Index. The PASI is a system used for assessing and grading the severity of psoriatic lesions and their response to therapy. The PASI produces a numeric score that can range from 0 to 72. PASI 75 is defined as a ≥75% improvement in PASI score from baseline. PASI 90 is defined as a ≥90% improvement in PASI score from baseline.
†PASI 75 response was evaluated in patients with ≥3% BSA psoriasis skin involvement at baseline.
‡Improvement from baseline in PASI is based on imputed data using LOCF for missing data.
Study design: GO-VIBRANT was a global, multicenter, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of SIMPONI ARIA® compared with placebo in 480 adult patients with active PsA. The target study population was biologic-naïve patients with active PsA for ≥6 months who met ClASsification criteria for Psoriatic ARthritis (CASPAR) criteria at screening. Patients in this trial had a diagnosis of PsA for at least 6 months and had symptoms of active disease (≥5 swollen joints and ≥5 tender joints and a CRP level of ≥0.6 mg/dL). At Week 0, patients were randomized in a 1:1 ratio to 1 of 2 treatment groups. Patients were allowed to be treated with or without MTX. Patients in the placebo group (n=239) were randomized to receive IV placebo infusions at Weeks 0, 4, 12, and 20. Patients in the SIMPONI ARIA® group (n=241) were randomized to receive SIMPONI ARIA® 2 mg/kg infusions at Weeks 0, 4, and q8w thereafter through Week 52. Patients were to receive a placebo infusion at Week 24 to maintain the treatment blind. At Week 24, all patients switched to treatment with SIMPONI ARIA® 2 mg/kg and were to receive administrations at Weeks 24, 28, and q8w through Week 52. The primary endpoint was the percentage of patients achieving an ACR20 response at Week 14.
ACR20 Response at Week 14 (primary endpoint): 75% of patients receiving SIMPONI ARIA® +/- MTX achieved ACR20 response vs 22% of patients receiving placebo +/- MTX (P<0.001)1-3
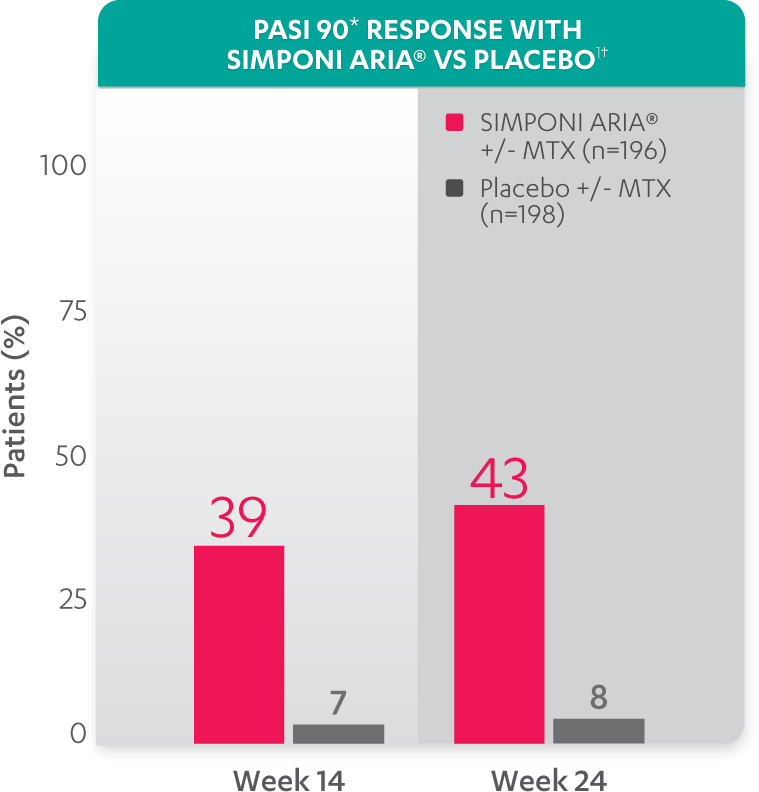
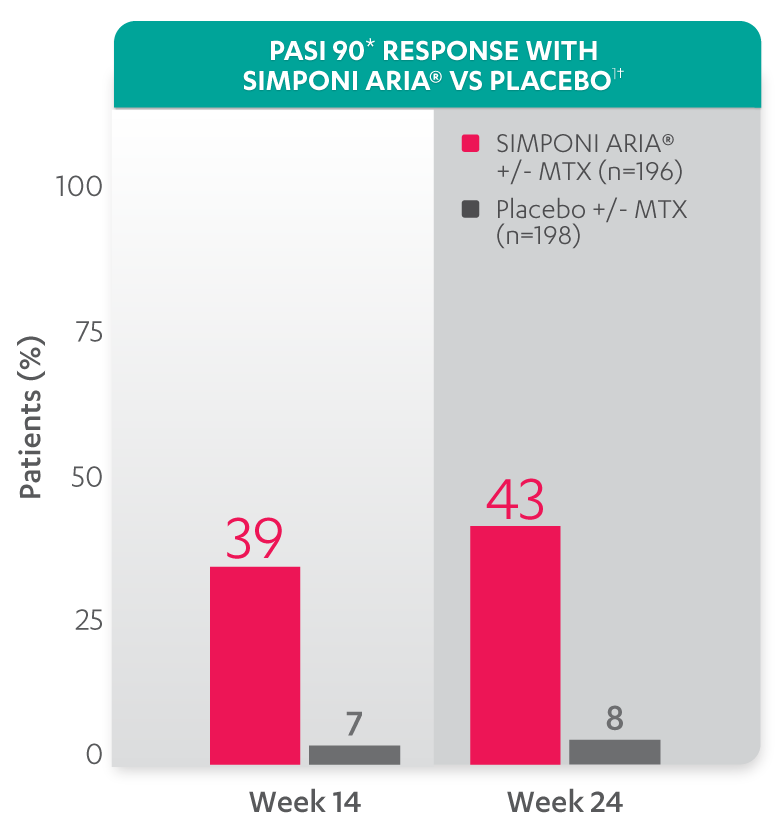
PASI response with SIMPONI ARIA® vs placebo
SIMPONI ARIA® has not been studied in and is not indicated for the treatment of plaque psoriasis. The GO-VIBRANT study population included patients with wide ranges of psoriasis disease severity not reflective of a plaque psoriasis population. These data do not provide evidence of a clinically meaningful improvement in mild to moderate psoriasis.
PASI 90 at Week 14 and Week 24 was not adjusted for multiplicity. Therefore, statistical significance has not been established.
*PASI 90 response was evaluated in patients with ≥3% BSA psoriasis skin involvement at baseline.
†Improvement from baseline in PASI is based on imputed data using LOCF for missing data.
Study design: GO-VIBRANT was a global, multicenter, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of SIMPONI ARIA® compared with placebo in 480 adult patients with active PsA. The target study population was biologic-naïve patients with active PsA for ≥6 months who met ClASsification criteria for Psoriatic ARthritis (CASPAR) criteria at screening. Patients in this trial had a diagnosis of PsA for at least 6 months and had symptoms of active disease (≥5 swollen joints and ≥5 tender joints and a CRP level of ≥0.6 mg/dL). At Week 0, patients were randomized in a 1:1 ratio to 1 of 2 treatment groups. Patients were allowed to be treated with or without MTX. Patients in the placebo group (n=239) were randomized to receive IV placebo infusions at Weeks 0, 4, 12, and 20. Patients in the SIMPONI ARIA® group (n=241) were randomized to receive SIMPONI ARIA® 2 mg/kg infusions at Weeks 0, 4, and q8w thereafter through Week 52. Patients were to receive a placebo infusion at Week 24 to maintain the treatment blind. At Week 24, all patients switched to treatment with SIMPONI ARIA® 2 mg/kg and were to receive administrations at Weeks 24, 28, and q8w through Week 52. The primary endpoint was the percentage of patients achieving an ACR20 response at Week 14.